On December 15, 2023, Pfizer and Astellas disclosed that the U.S. Food and Drug Administration (FDA) granted approval for PADCEV® (enfortumab vedotin-ejfv), an antibody-drug conjugate (ADC), in conjunction with KEYTRUDA® (pembrolizumab), a PD-1 inhibitor for the treatment of adult patients with locally advanced or metastatic urothelial cancer (la/mUC). Notably, this combination is the first approved treatment providing an alternative to platinum-containing chemotherapy, which currently serves as the standard of care in first-line la/mUC. [1]
Enfortumab vedotin (PADCEV) is a Nectin-4 directed ADC developed by Astellas and Seattle. In December 2019, the FDA granted accelerated approval to enfortumab vedotin-ejfv (PADCEV) for the treatment of patients with la/mUC who have previously received a programmed cell death protein 1 or programmed death ligand 1 inhibitor, and a platinum-containing chemotherapy in the neoadjuvant/adjuvant, locally advanced or metastatic setting. [2] Since then, based on the results of the validation clinical phase III (EV-301) study, its monotherapy has been approved for marketing in multiple countries.

Figure 1. Enfortumab vedotin (PADCEV)
In addition to monotherapy, Astellas has been actively exploring the combination of enfortumab vedotin with the PD-1 inhibitor pembrolizumab, and has achieved quite excellent results.
On April 20, 2023, the FDA granted accelerated approval for combination therapy (PADCEV & KEYTRUDA) for the treatment of adult patients with la/mUC who are not eligible to receive cisplatin-containing chemotherapy. This approval was based on the dose-escalation/Cohort A and Cohort K of the KEYNOTE-869 (EV-103) study. In these EV-103 cohorts, patients treated with PADCEV in combination with pembrolizumab (n=121) obtained a 68% confirmed ORR (95% CI: 58.7 to 76.0) with 12% of patients experiencing a complete response(CR) and 55% of patients experiencing a partial response (PR). The median DOR per BICR for Dose Escalation/Cohort A was 22.1 months (range: 1.0+ to 46.3+) and was not reached (range: 1.2 to 24.1+) for Cohort K. The median number of treatment cycles (per 21-day treatment cycle) was nine in Dose Escalation/Cohort A and 11 in Cohort K. [3]
On December 15, 2023, the combination therapy received full FDA approval for the first-line treatment of la/mUC based on the outstanding results of the clinical Phase III study (EV-302/KN-A39). The results showed statistically significant improvements in OS and PFS in patients receiving Padcev in combination with Keytruda compared to platinum-containing chemotherapy regimens. Median OS was 31.5 months (95% CI: 25.4- not estimable) for patients receiving Padcev combination therapy and 16.1 months (95% CI: 13.9-18.3) for patients treated with platinum-containing chemotherapy, namely a 53% reduction in the risk of death for patients in the combination therapy group compared to the chemotherapy group (HR: 0.47, 95% CI: 0.38- 0.58, p<0.0001).

Figure 2. EV-302 OS
In addition, the median PFS was 12.5 months (95% CI: 10.4-16.6) for patients receiving Padcev combination therapy and 6.3 months (95% CI: 6.2-6.5) for patients receiving platinum-containing chemotherapy, meaning that patients in the combination therapy group had a significantly prolonged PFS and a 55% lower risk of disease progression or death compared to chemotherapy (HR: 0.45 95% CI: 0.38-0.54, p<0.0001). [1]

Figure 3. EV-302 PFS
PD-1 & ADC Combination Therapy in Clinical Trails
Several combination therapies of ADCs and PD-1 inhibitors have already made an impact on the first-line treatment of certain tumors. Currently, there are many combination therapies in clinical for both solid tumors and hematologic malignancies, and some have demonstrated strong data in early clinical trials.
EVOKE-02
EVOKE-02 is a multi-cohort Phase II study exploring the safety and treatment activity sacituzumab govitecan (SG) plus pembrolizumab (pembro) with or without platinum chemotherapy in first-line metastatic non–small cell lung cancer (NSCLC).
The preliminary results of Cohort A and Cohort B were reported at WCLC 2023. Patients in Cohort A had a PD-L1 TPS ≥ 50% and patients in Cohort B had a PD-L1 TPS < 50%, and both cohorts were treated with Trodelvy in combination with KEYTRUDA. In Cohort A (n=29), the confirmed and unconfirmed objective response rate (ORR) was 69%, and disease control rate (DCR) was 86%. In Cohort B (n=32), confirmed and unconfirmed ORR was 44%, and DCR was 78%. Across both cohorts, the ORR was 56%, and DCR was 82%. Median duration of response (DoR) was not reached at the time of data cut-off, and DoR rate at six months was 88% in both cohorts. [4]
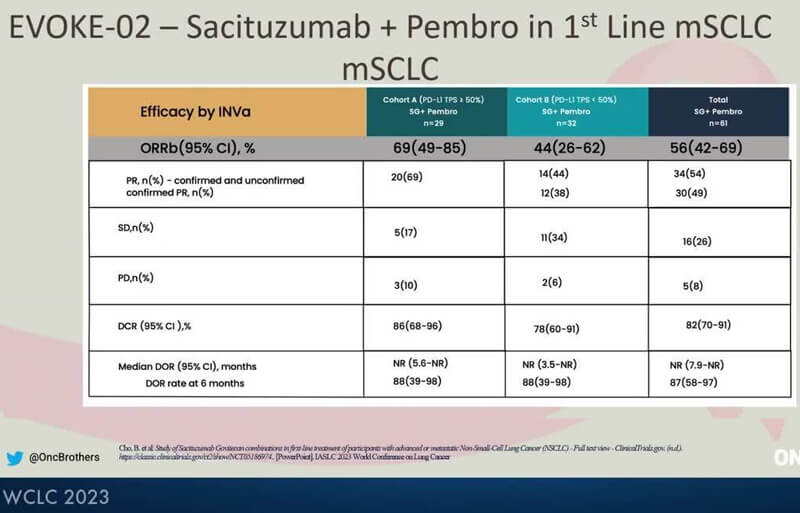
Figure 4. EVOKE-02 ORR
The first-line treatment of mNSCLC with sacituzumab govitecan in combination with pembrolizumab has initially shown encouraging antitumor efficacy regardless of PD-L1 expression status, and the ORR result of 69% in the PD-L1 high-expression group has also released an even more positive signal. The randomized phase III EVOKE-03 study is currently underway and will provide confirmatory evidence for the first-line use of this novel regimen in the PD-L1 TPS ≥ 50% population.
TROPION-Lung02
TROPION-Lung02 is a multi-cohort Phase Ib study exploring the safety and treatment activity of datopotamab deruxtecan (Dato-DXd) in combination with pembrolizumab with or without platinum chemotherapy in participants with aNSCLC.
Updated results from the 2023 ASCO Congress showed that in the first-line treatment population, the ORR for patients treated with the doublet therapy (n = 34) and triplet therapy (n = 53) regimens was 50% and 57%, respectively, with a DCR of 91% in both cases, and the median DOR has not yet been reached. Among all patients, the doublet therapy (n = 61) had an ORR of 38%, a DCR of 84%, and a preliminary median progression-free survival (PFS) of 8.3 months, while the triplet therapy (n = 71) had an ORR of 49%, a DCR of 87%, and a preliminary median PFS of 7.8 months, with a median DOR that has not yet been reached [5].

Figure 5. TROPION-Lung02 2023 ASCO results
The Phase III TROPION-Lung07 and TROPION-Lung08 studies are currently underway to further confirm the efficacy of the first-line treatment of the regimen.
SGN35-027
In June 2023, Seagen announced updated efficacy and safety results from Part C of a phase 2 single-arm trial (SGN35-027) evaluating a novel combination of brentuximab vedotin (Adcetris), nivolumab (Opdivo), dacarbazine, and doxorubicin in patients across multiple stages of Hodgkin lymphoma. The results showed an overall response rate (ORR) of 98% and a complete response (CR) of 93% in patients who received this combination therapy. [6]
Suppose the excellent results of this trial are successfully translated into an OS benefit and carried over into Phase III. In that case, there is a strong probability that the standard of care for first-line treatment of cHL will be rewritten.
Conclusion
The combination therapy of ADC with PD-1 inhibitors has demonstrated immense potential in the field of cancer treatment, offering patients a broader spectrum of therapeutic options. We look forward to achieving more encouraging clinical results in the future, providing cancer patients with increasingly innovative and effective treatment approaches.
References:
[1] https://www.pfizer.com/news/press-release/press-release-detail/padcevr-enfortumab-vedotin-ejfv-keytrudar-pembrolizumab
[2] Chang E, Weinstock C, Zhang L, et al. FDA Approval Summary: Enfortumab Vedotin for Locally Advanced or Metastatic Urothelial Carcinoma. Clin Cancer Res. 2021;27(4):922-927. doi:10.1158/1078-0432.CCR-20-2275
[3] https://www.astellas.com/en/news/27486 FDA Grants Accelerated Approval for PADCEV® (enfortumab vedotin-ejfv) with KEYTRUDA® (pembrolizumab) for First-Line Treatment of Locally Advanced or Metastatic Urothelial Cancer
[4] https://www.gilead.com/news-and-press/press-room/press-releases/2023/9/gileads-phase-2-evoke02-study-of-trodelvy-sacituzumab-govitecanhziy-in-combination-with-keytruda-pembrolizumab-demonstrates-promising-clinica
[5] Yasushi Goto, et al. TROPION-Lung02: Datopotamab deruxtecan (Dato-DXd) plus pembrolizumab (pembro) with or without platinum chemotherapy (Pt-CT) in advanced non-small cell lung cancer (aNSCLC). 2023 ASCO. Abstract 9004.
[6] https://www.biospace.com/article/releases/seagen-announces-adcetris-brentuximab-vedotin-plus-novel-immunotherapy-combination-delivers-98-percent-overall-response-rate-and-93-percent-complete-response-rate-in-patients-with-early-stage-classical-hodgkin-lymphoma-chl-/

