RNA interference (RNAi) is a naturally occurring cellular mechanism that regulates gene expression, enabling cells to defend against the invasion of foreign nucleic acids and control gene expression. The key molecules involved in RNAi are small interfering RNA (siRNA), microRNA (miRNA), and PIWI-interacting RNA (piRNA).
In 1998, Andrew Fire and Craig Mello discovered the phenomenon of post-transcriptional gene silencing in the nematode worm Caenorhabditis elegans, which they termed RNA interference. In 1999, David Baulcombe first identified siRNA in plants and demonstrated its role in RNAi by conducting experiments with cell extracts to verify the enzymatic activity of siRNA, showing that siRNA can silence specific genes in mammalian cells.
siRNA Mechanism of Action
siRNA is a class of short double-stranded RNA molecules with a length of 21 to 25 nucleotides. The mechanism of siRNA action involves two main stages:

Figure 1. siRNA Mechanism of Action [1]
Initiation Stage (First Phase):
- ● Longer double-stranded RNA (dsRNA) or short hairpin RNA (shRNA) is present.
- ● An enzyme called Dicer cleaves this dsRNA or shRNA into smaller fragments, generating siRNA molecules.
Effector Stage (Second Phase):
- ● The mature siRNA combines with proteins, including Argonaute-2 (AGO2), to form the RNA-induced silencing complex (RISC).
- ● During RISC assembly, AGO2 separates the siRNA duplex into two single strands: the guide strand and the passenger strand.
- ● The passenger strand will be cleaved, while the guide strand is retained and served as a guide to conduct the alignment with the target mRNA sequence.
- ● The guide strand within the RISC complex pairs with the complementary mRNA.
- ● The RISC complex, with the help of endonuclease activity, cleaves the target mRNA, preventing its translation into proteins.
This targeted mRNA cleavage leads to the suppression of gene expression, providing a powerful mechanism for controlling gene activity in cells.
Advantages of siRNA Therapeutics
After more than two decades of research, siRNA therapeutics has been widely acknowledged as a precision medicine therapy. Currently, there are six siRNA therapeutics approved by FDA (patisiran, givosiran, lumasiran, inclisiran, vutrisiran, and Rivfloza) for clinical use, and about 20 additional candidates have advanced to the late stages of clinical investigation. siRNA therapy exhibits a series of advantages compared to other treatment modalities.
(1) siRNA exhibits a high level of safety. As it acts on the post-translational stage of gene expression, it does not interact with DNA, thereby avoiding the common risks of mutations and teratogenic effects associated with gene therapy.
(2) siRNA gene silencing is highly efficient. In comparison to small molecule therapies and monoclonal antibodies, siRNA has inherent advantages. This is because siRNA executes its function by completing Watson-Crick base pairing with mRNA, whereas small molecules and monoclonal antibody drugs require recognition of the protein's 3D spatial conformation. Evaluation indicates that randomly designed siRNA has a probability of 11% to 18% of achieving a silencing effect of 90% to 95%, and a probability of 58% to 78% of achieving a 50% silencing effect.
(3) siRNA demonstrates high specificity. It can only silence homologous genes, leaving unrelated genes unaffected. Moreover, a change in just one nucleotide within the 19 to 21 nucleotide-pairing region of the siRNA sequence can eliminate the silencing effect on the target gene.
(4) siRNA is multifunctional. Through sequence design, siRNA can target various genes, making it applicable for the treatment of diverse diseases.
(5) siRNA is easy to synthesize, with low production costs. The chemical synthesis of siRNA is straightforward, and unlike proteins or antibodies, siRNA does not require laborious steps such as expression and purification, resulting in lower production costs.
Three Challenges of siRNA Clinical Investigation
Despite the significant progress that has been made in siRNA drug research, some critical challenges remain that should be overcome. Specifically, targeting accumulation and cellular uptake (Entry), endosomal and lysosomal escape (Escape), and in vivo drug pharmaceutical performance (Efficacy) are the three pivotal issues that limit the clinical translation and application of siRNA. [2]
Entry challenge: targeted accumulation and cellular uptake
The first challenge is the effective accumulation of siRNA in target organs/tissues and effective internalization into the target cells (Figure 2). siRNA encounters risks of clearance in the body:
- ● Nanocarrier-encapsulated siRNAs typically bind with serum proteins, subsequently leading to uptake by the reticuloendothelial system (RES) and phagocytic clearance.
- ● siRNA can be rapidly degraded by nucleases or phosphatases in plasma, tissues, and the cytoplasm.
- ● siRNA struggles to reach target cells: It must traverse the endothelium of capillaries to enter target tissues, but due to extensive adhesion and tight junctions, as well as effective barriers like the blood-brain barrier (BBB) and blood-retinal barrier, reaching target cells is challenging.
- ● Additionally, in solid tumors, due to the enhanced permeability and retention (EPR) effect, siRNA may passively accumulate in the liver or tumor tissues.

Figure 2. The entry challenge encompasses the fate of siRNA therapeutics in the vascular system. [2]
Escape challenge: endosomal and lysosomal escape
The second challenge lies in achieving effective escape from endosomes and lysosomes. Despite cellular uptake via endocytosis, less than 1% of siRNAs can escape from the endosome, with a passive escape rate below 0.01%.
While the asialoglycoprotein receptor (ASGPR) offers promise, particularly in liver cells, where it's abundant, achieving therapeutic levels in hepatocytes with GalNAc-siRNA conjugates is feasible. However, this solution remains elusive for other cell types due to lower receptor expression levels and longer recycling times.
Attempts to enhance endosomal escape using various methods like modified pH sensitivity and ion-penetrating agents have not fully overcome this challenge. Though entrapped RNA therapeutics in endosomes sustain long responses, a significant portion fails to reach the cytoplasm. This balance between sustained release and effective cytoplasmic penetration is critical for broader application in treating human diseases.

Figure 3. The ‘escape’ challenge is the insufficient endosomal escape of siRNA. [2]
Efficacy challenge: in vivo pharmaceutical performance
The third challenge is that siRNA requires good stability, lasting effectiveness, and safety in the body. There are risks associated with delivery systems. The use of viral vectors to deliver nucleic acids in vivo has certain toxic side effects, primarily limited to preclinical research at present. In clinical settings, chemical synthetic delivery systems, such as cationic lipids and most inorganic nanoparticles, are mainly utilized, but there is a risk of inducing cell apoptosis and inflammation. Additionally, delivery systems in the biological organism must ensure ease of production, quality control, and transport to enable large-scale clinical applications.
A preclinical toxicity evaluation model for RNA drugs has not been established. Due to limited overlap between non-human primates (NHP) and the human genome sequence, dose-response relationships obtained from mouse models cannot be directly applied to humans, making it challenging to predict efficacy. It may be possible to expand the use of non-human primates as models or select organ-like models relevant to the disease for evaluation.
Furthermore, exogenous oligonucleotides may exhibit immunogenicity, triggering immune responses. With technological breakthroughs, chemical modifications have been widely applied to enhance the performance of siRNA, such as improving stability, reducing/eliminating off-target effects, and minimizing immunogenicity. Although chemical modifications have been successful in achieving low-dose, long-lasting gene silencing, challenges still exist, such as off-target-induced toxicity.
Therefore, the toxicity and immunogenicity of siRNA drugs need careful evaluation in both preclinical and clinical research.
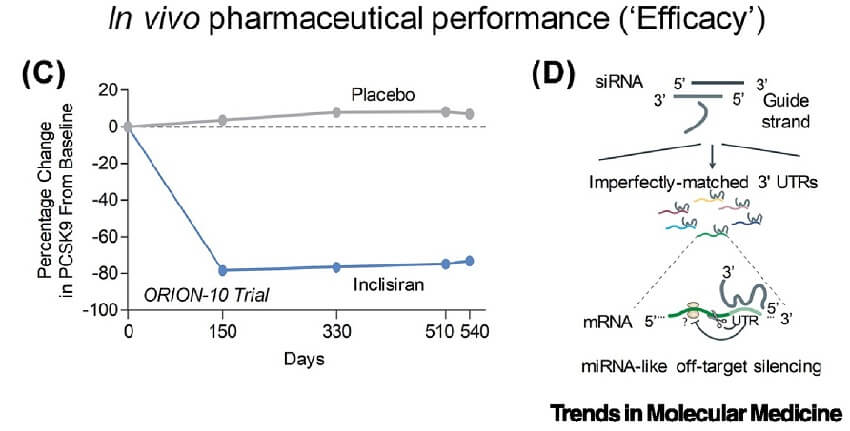
Figure 4. The ‘efficacy’ challenge encompasses the pharmaceutical performance of siRNAs in vivo. [2]
Strategies to Overcome These Challenges
To address these challenges and advance the development of siRNA therapy, the following are four strategies worth exploring.
Developing New Chemical Modification Methods: Optimizing chemical modifications can enhance the stability, specificity, safety, and bioavailability of siRNA. This involves the development of new chemical modification monomers, modification patterns, and RNAi-triggering structures.
Establishing Unique Delivery Systems: Lipid Nanoparticles (LNPs) and GalNAc have become mature delivery systems widely used in siRNA therapies. Conjugate molecules, exosomes, and cell-penetrating peptides (CPPs) as emerging systems have shown promise. In the future, researchers may explore more targeted and effective siRNA drug delivery systems.
Expanding Disease Targets: While siRNA technology is primarily used to target protein-coding genes, recent studies indicate that non-coding RNAs (ncRNAs) play crucial roles in various diseases. Targeting ncRNAs may cover a broader range of diseases, offering improved therapeutic options. Additionally, exploring the interactions between coding RNA and ncRNA can provide new insights into disease mechanisms and facilitate more effective therapeutic interventions. Besides regulating gene expression at the mRNA level, siRNA technology can also target epigenetic modifications such as DNA methylation or histone modification, which play crucial roles in the development and progression of diseases. By modulating epigenetic marks, it may be possible to reprogram gene expression patterns and reverse disease phenotypes.
Exploring Combination Therapies: Employing a dual-targeting approach by combining siRNA with other therapeutic agents like chemotherapy drugs, antibodies, or immune modulators holds the potential to enhance efficacy, overcome resistance, and reduce off-target effects.
In the coming years, as researchers continue to explore these promising research areas, we can expect to witness breakthroughs in the field of siRNA drug development. Ultimately, these advancements may lead to novel and more effective treatments for various diseases.
References:
[1] de Brito e Cunha, D.; Frederico, A.B.T.; Azamor, T.; Melgaço, J.G.; da Costa Neves, P.C.; Bom, A.P.D.A.; Tilli, T.M.; Missailidis, S. Biotechnological Evolution of siRNA Molecules: From Bench Tool to the Refined Drug. Pharmaceuticals 2022, 15, 575. https://doi.org/10.3390/ph15050575
[2] Guo S, Zhang M, Huang Y. Three 'E' challenges for siRNA drug development. Trends Mol Med. 2024;30(1):13-24. doi:10.1016/j.molmed.2023.10.005

