In 2023, the Center for Drug Evaluation and Research (CDER) of the U.S. Food and Drug Administration (FDA) approved a total of 55 new drugs, including 38 new molecular entities (NMEs) and 17 biologics license applications (BLAs). This marks a five-year high, second only to the 59 drugs approved in 2018.

Fig. 1 | 30 years of novel FDA approvals. [1]
Then, what is the anticipated number of drug approvals in 2024? Evaluate recently published a report forecasting the top 10 most anticipated drug launches of 2024.

Fig. 2 | Top 10 most anticipated drug launches of 2024 [2]
Biggest Potential Launches of 2024
NO.1 KarXT(xanomeline-trospium)
- ▶ Company: Bristol Myers Squibb/Karuna Therapeutics
- ▶ Indication: Psychiatric and neurological conditions
- ▶ Status: PDUFA date of September 26, 2024
KarXT (xanomeline-trospium), devloped by Karuna Therapeutics, is an oral, investigational M1/M4-preferring muscarinic agonist in development for the treatment of psychiatric and neurological conditions, including schizophrenia and psychosis in Alzheimer's disease, etc.
KarXT stands out as a first-in-class medication, presenting an entirely innovative dual mechanism of action. Unlike current therapies, KarXT does not rely on the dopaminergic or serotonergic pathways, and it is designed to harness the therapeutic potential of xanomeline while managing peripheral side effects through trospium. This innovative approach has the potential to offer a distinct therapy and, upon approval, could positively influence the lives of numerous individuals suffering from serious mental illness.
In December 2023, Bristol Myers Squibb (BMS) signed a definitive agreement for the acquisition of Karuna Therapeutics for an equity value totalling $14bn in cash. Through the transaction, BMS gained access to Karuna’s lead asset KarXT. The U.S. FDA has accepted New Drug Application (NDA) for KarXT with PDUFA date of September 26, 2024. If approved, KarXT would represent the first new pharmacological approach to treating schizophrenia in several decades.

Fig. 3 | KarXT, source: Karuna Therapeutics official website
NO. 2. Donanemab
▶ Company: Eli Lilly
▶ Indication: Alzheimer's disease
▶ Status: FDA review results expected in Q1 2024
Donanemab is a humanized antibody and acts against the N-truncated pyroglutamate amyloid-β peptide at position 3 (pGlu3-Aβ, AβpE3) and is currently being investigated as a treatment for Alzheimer's disease. pGlu-3 Aβ is an N-terminally truncated and post-translationally modified Aβ species found in Alzheimer's disease (AD) brain. Its increased peptide aggregation propensity and toxicity make it an attractive emerging treatment strategy for AD.
In July 2023, Eli Lilly announced at the 2023 Alzheimer's Association International Conference (AAIC) that the Phase 3 clinical trial TRAILBLAZER-ALZ 2 for Donanemab achieved its primary endpoint and all secondary endpoints. In early-stage AD patients with low/intermediate-level tau pathology, based on the Integrated Alzheimer's Disease Rating Scale (iADRS) scores, Donanemab treatment reduced the risk of disease progression by 35.1% compared to placebo; in the overall population, Donanemab treatment lowered the risk of disease progression by 22.3%. Additionally, among early-stage AD patients with low/intermediate-level tau pathology, 47% of patients receiving Donanemab treatment maintained stable conditions after one year, whereas only 29% of patients receiving placebo treatment remained stable.

Fig. 4 | Changes in iADRS and CDR-SB from baseline to week 76. [3]
Eli Lilly has completed its FDA submission for full approval of donanemab. The FDA is expected to announce its approval decision for this drug in early 2024.
NO. 3. Resmetirom
▶ Company: Madrigal Pharmaceuticals
▶ Indication: Nonalcoholic steatohepatitis (NASH)
▶ Status: Approved
Resmetirom, or MGL-3196, is a first-in-class oral thyroid hormone receptor-beta (THRβ) agonist in clinical development for the treatment of NASH. THRβ is the main receptor for thyroid hormones in the liver and plays an essential role in lipid metabolism. In studies, THR-β agonism has shown the potential to reduce inflammatory liver fat and fibrosis while lowering cholesterol and other atherogenic lipids.
The FDA has accepted for review its NDA for resmetirom for the treatment of adult patients with NASH with liver fibrosis and has granted Priority Review and assigned a PDUFA date for resmetirom of March 14, 2024.
Madrigal is currently conducting four Phase 3 clinical trials to demonstrate the safety and efficacy of resmetirom for the treatment of NASH: MAESTRO-NASH, MAESTRO-NAFLD-1, MAESTRO-NAFLD-OLE, and MAESTRO-NASH-OUTCOMES.

Fig. 5 | MAESTRO-NASH Dual Primary Endpoints (Week 52) [4]
NO. 4. Sotatercept
▶ Company: Merck
▶ Indication: Pulmonary Arterial Hypertension (PAH)
▶ Status: PDUFA date of 26 March, 2024
Sotatercept is an investigational and potential first-in-class activin receptor type IIA-Fc (ActRIIA-Fc) fusion protein designed for the treatment of pulmonary arterial hypertension in adult patients. Comprising the Fc domain of human IgG linked to the extracellular domain of human ActRIIA, it functions as a ligand trap for selected TGF-β superfamily members. Inhibition of these ligands by sotatercept is proposed to rebalance pulmonary vascular homeostasis toward growth-inhibiting and proapoptotic signaling.
Sotatercept achieved the primary endpoints in the Phase 3 clinical trial STELLAR. In comparison to a placebo, it demonstrated a statistically significant and clinically meaningful improvement in the 6-minute walk distance (6MWD) for patients. It has received breakthrough therapy designation from the FDA, making it the first investigational therapy for PAH to receive such designation.

Fig. 6 | Changes in the 6-minute walk distance (6MWD) through week 24 [5]
NO. 5. Datopotamab deruxtecan
▶ Company: Daiichi Sankyo & AstraZeneca
▶ Indication: Lung & Breast Cancer
▶ Status: FDA review results expected in 2024
Datopotamab deruxtecan (Dato-DXd) is an antibody-drug conjugate (ADC) targeting TROP2, jointly developed by AstraZeneca and Daiichi Sankyo. Dato-DXd is composed of a humanized anti-TROP2 IgG1 monoclonal antibody linked to a cleavable tetrapeptide linker, connected to a topoisomerase I inhibitor payload (a derivative of camptothecin).

Fig. 7 | Datopotamab deruxtecan
In October 2023, trial results presented at the European Society for Medical Oncology (ESMO) Congress revealed that, compared to active drugs, Dato-DXd therapy significantly reduced the risk of disease progression or death in patients with advanced non-small cell lung cancer (NSCLC) and inoperable or metastatic HR-positive, HER2-low-expressing, or HER2-negative breast cancer in two separate Phase 3 trials. [6]
NO. 6. Acoramidis
▶ Company: BridgeBio Pharma
▶ Indication: ATTR-CM
▶ Status: Submitted an NDA to FDA in December 2023
Acoramidis is an investigational, next-generation, orally-administered, highly potent, small molecule stabilizer of transthyretin (TTR) for the treatment of patients with transthyretin amyloid cardiomyopathy (ATTR-CM). It is designed to mimic protective T119M mutation by stabilizing TTR tetramers to slow or halt disease progression. On December 4, 2023, BridgeBio Pharma, Inc. announced the submission of a New Drug Application (NDA) to the U.S. FDA for acoramidis to treat ATTR-CM. The application is based on the positive results from the ATTRibute-CM.
In July 2023, BridgeBio announced positive topline results from ATTRibute-CM, and in August 2023, BridgeBio presented detailed positive results at the European Society of Cardiology Congress 2023. An 81% on-treatment survival rate (versus a 74% survival rate on placebo) was observed, which begins to approach actuarial models of life expectancy absent ATTR-CM (85% in this population as has been documented). Similarly, the annualized cardiovascular (CV)-related hospitalization rate of 0.29 in the acoramidis arm was observed, similar to the 0.26 overall hospitalization rate reported by the U.S. Department of Health and Human Services for the general Medicare population. The absolute risk reduction was 6.43% and the relative risk reduction was 25%. [7]
NO. 7. mRNA-1345
▶ Company: Moderna
▶ Indication: Pre-Registration for Respiratory Syncytial Virus (RSV) Infections
▶ Status: Submitted marketing authorization applications with global regulators
mRNA-1345 is an investigational RSV vaccine that consists of a single mRNA sequence encoding for a stabilized prefusion F glycoprotein. The vaccine uses the same lipid nanoparticles (LNPs) as in the Moderna COVID-19 vaccines. The F glycoprotein is on the surface of the virus and is required for infection by helping the virus to enter host cells. It exists in two states, prefusion and postfusion. The prefusion conformation is a significant target of potent neutralizing antibodies, and the protein sequences are largely similar across both RSV-A and RSV-B subtypes.
The key trials of mRNA-1345 achieved two primary endpoints, with vaccine efficacy of 83.7% against RSV lower respiratory tract disease (RSV-LRTD) defined by two or more symptoms, and 82.4% against RSV-LRTD defined by three or more symptoms. The U.S. FDA has granted mRNA-1345 fast track designation and breakthrough therapy designation to assist adults aged 60 or above in preventing RSV-LRTD and acute respiratory disease (ARD). [8]
NO. 8. Anktiva (N-803)
▶ Company: ImmunityBio
▶ Indication: BCG-unresponsive non-muscle-invasive bladder cancer (NMIBC) carcinoma in situ (CIS)
▶ Status: PDUFA date of 23 April 2024
Anktiva™ (ImmunityBio's lead cytokine infusion protein) is a novel interleukin-15 (IL-15) superagonist complex and has received Breakthrough Therapy and Fast Track Designations from the U.S. Food and Drug Administration (FDA) for BCG-unresponsive CIS non-muscle invasive bladder cancer (NMIBC).
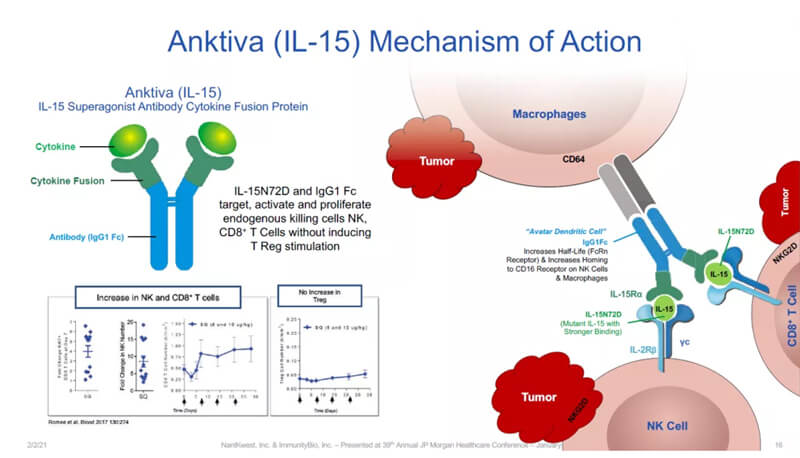
Fig. 8 | Anktiva Mechanism of action
Anktiva is a crucial player in the immune system that influences the development, maintenance, and function of natural killer (NK) cells and immune T cells. Comprising the IL-15 mutant (IL-15N72D) bound to the IL-15 receptor alpha/IgG1 Fc fusion protein, Anktiva directly and specifically stimulates CD8-positive T cells and NK cells by binding to the βγT cell receptor while avoiding the stimulation of regulatory T cells (Treg). Compared to natural, non-complexed IL-15, Anktiva demonstrates superior pharmacokinetic properties in patients, exhibiting prolonged presence in lymphoid tissues and enhanced anti-tumor activity. In a Phase 2/3 clinical trial treating BCG-unresponsive NMIBC in situ (CIS), Anktiva, when combined with BCG, achieved the primary endpoint for patients with the papillary tumor subtype, with 57% of patients reaching a disease-free survival (DFS) of 12 months. [9]
NO. 9. Ensifentrine
▶ Company: Verona Pharma
▶ Indication: Chronic obstructive pulmonary disease (COPD)
▶ Status: PDUFA date of 26 June 2024
Ensifentrine is a first-in-class, selective, dual phosphodiesterase (PDE)3 and PDE4 inhibitor with bronchodilator and antiinflammatory effects. Verona Pharma initiates phase 3 clinical trials, ENHANCE-1 and ENHANCE-2, with nebulized ensifentrine for the maintenance treatment of chronic obstructive pulmonary disease (COPD). Ensifentrine met the primary endpoints in both trials, showing statistically significant and clinically meaningful improvements in patient's lung function. A combined analysis of data from ENHANCE-1 and ENHANCE-2 demonstrated a substantial reduction in the risk of COPD exacerbation with ensifentrine. If approved, ensifentrine is expected to be the first novel mechanism available for the treatment of COPD in more than 10 years.
NO. 10. Imetelstat
▶ Company: Geron
▶ Indication: Lower risk myelodysplastic syndromes (MDS)
▶ Status: PDUFA date of 16 June 2024
Imetelstat is a first-in class telomerase inhibitor for the treatment of transfusion-dependent anemia in patients with lower-risk myelodysplastic syndromes (MDS).
The NDA submission is based on results from IMerge Phase 3, in which the primary endpoint of 8-week transfusion independence (TI) was significantly higher with imetelstat vs. placebo (p<0.001), with median TI duration approaching one year for imetelstat 8-week TI responders. Mean hemoglobin levels in imetelstat-treated patients increased significantly (p<0.001) over time compared to placebo patients. Statistically significant and clinically meaningful efficacy results were achieved across key MDS subgroups irrespective of ring sideroblast (RS) status, baseline transfusion burden and IPSS risk category. Patient-reported outcomes (PRO) data reported a sustained meaningful improvement in fatigue for imetelstat-treated patients vs. placebo. Safety results were consistent with prior imetelstat clinical experience.

Fig 9. | Top-line data from Phase 3 clinical trial of Imetelstat [10]
References:
[1] 2023 FDA approvals, https://www.nature.com/articles/d41573-024-00001-x
[2] Will biopharma find its footing? 2024 Preview. Retrieved December 29, 2023 from https://www.evaluate.com/thought-leadership/pharma-reports/2024-preview-report?utm_source=website&utm_medium=banner&utm_campaign=2024_preview&utm_content=ebook
[3] Sims JR, Zimmer JA, Evans CD, et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. 2023;330(6):512–527. doi:10.1001/jama.2023.13239
[4] Primary Results From MAESTRO-NASH: A Pivotal Phase 3 52-week Serial Liver Biopsy Study in 966 Patients With NASH & Fibrosis. Retrieved November 10, 2023 from https://ir.madrigalpharma.com/static-files/e8a35f47-f841-49d4-9c21-781f41177609
[5] Merck Announces Positive Top-line Results from Pivotal Phase 3 STELLAR Trial Evaluating Sotatercept for the Treatment of Adults with Pulmonary Arterial Hypertension (PAH) https://www.merck.com/news/merck-announces-positive-top-line-results-from-pivotal-phase-3-stellar-trial-evaluating-sotatercept-for-the-treatment-of-adults-with-pulmonary-arterial-hypertension-pah/
[6] Datopotamab deruxtecan met the PFS endpoint in previously treated NSCLC
https://dailyreporter.esmo.org/esmo-congress-2023/non-small-cell-lung-cancer/datopotamab-deruxtecan-met-the-pfs-endpoint-in-previously-treated-nsclc
[7] BridgeBio Pharma Announces Submission of New Drug Application (NDA) to U.S. Food and Drug Administration (FDA) for Acoramidis for the Treatment of Patients with Transthyretin Amyloid Cardiomyopathy (ATTR-CM). Retrieved December 6, 2023, from https://www.globenewswire.com/news-release/2023/12/05/2790824/0/en/BridgeBio-Pharma-Announces-Submission-of-New-Drug-Application-NDA-to-U-S-Food-and-Drug-Administration-FDA-for-Acoramidis-for-the-Treatment-of-Patients-with-Transthyretin-Amyloid-Ca.html
[8] Moderna Announces mRNA-1345, an Investigational Respiratory Syncytial Virus (RSV) Vaccine, Has Met Primary Efficacy Endpoints in Phase 3 Trial in Older Adults https://investors.modernatx.com/news/news-details/2023/Moderna-Announces-mRNA-1345-an-Investigational-Respiratory-Syncytial-Virus-RSV-Vaccine-Has-Met-Primary-Efficacy-Endpoints-in-Phase-3-Trial-in-Older-Adults/default.aspx#:~:text=Following%20review%20by%20an%20independent,by%20two%20or%20more%20symptoms.
[9] FDA accepts resubmission of BLA for N-803 in NMIBC https://www.urologytimes.com/view/fda-accepts-resubmission-of-bla-for-n-803-in-nmibc
[10] https://ir.geron.com/investors/press-releases/press-release-details/2023/Geron-Announces-FDA-Acceptance-of-New-Drug-Application-for-Imetelstat-for-the-Treatment-of-Lower-Risk-MDS/default.aspx Geron Announces FDA Acceptance of New Drug Application for Imetelstat for the Treatment of Lower Risk MDS
Related Articles:
Top 10 Projected Best-Selling Drugs in 2024

