Exosomes are natural nanoparticles that are widely distributed in tissues and can be produced by all known cells. Exosomes are nanoscale extracellular vesicles wrapped in lipid bilayer membranes, secreted by most eukaryotic cells, and have unique characteristics - inherent stability, low immunogenicity, biocompatibility and good biological membrane penetrating ability - allowing them to function as highly efficient natural nanocarriers. More and more studies have shown that exosomes can regulate a variety of biological functions, they are a vital source of biomarkers in clinical diagnosis.
1. Introduce of Exosomes
Exosomes are membranous vesicles released into the extracellular matrix after the fusion of multivesicular bodies (MVB) and cell membranes in cells, which can transport abundant proteins, lipids, DNA, RNA and other substances. In nature, exosomes protect and deliver functional macromolecules, including nucleic acids, proteins, lipids, and carbohydrates. Exosomes alter the behavior of recipient cells by transferring macromolecules to recipient cells or activating signaling pathways. Such as transcription and translation, tissue repair, immune balance, cell differentiation and regeneration, apoptosis, cell migration, metabolic regulation, and microbial environment, these are far from covering the extensive research work in academia in recent years.
Exosomes can be called a "generalist" of "both positive and evil", the occurrence and development of diseases cannot be separated from it, the immune function cannot be separated from it, disease diagnosis sometimes needs it, and it can also be used to make vaccines. More "bad" and "good" exosomes are still being discovered, and the role of more exosomes is constantly being explored. Recent studies have shown the potential use of exosomes as cell-free therapies, which may provide new strategies for solving difficult problems in the clinic.
More and more studies have shown that exosomes play a crucial role in long-distance communication between cells, because they can reach other cells and tissues through the circulatory system to produce remote regulation. As a result, great interest has been generated in the function of exosomes and their potential applications as small molecule therapeutic vectors. This article discusses the potential of exosomes as "natural nanoparticles" for the delivery of drugs and genes and compares its advantages and disadvantages with liposomes.

Figure 1 Intercellular communication via exosomes
2. Advantages And Potential of Exosomes As Carriers
To achieve drug or gene delivery, it is important to consider the type of vector used. Exosome carriers combine the advantages of cell-based drug delivery and nanotechnology for efficient drug delivery. Compared with cell therapy, exosomes are easier to store and can reduce safety risks. Exosomes can be isolated from patient fluids or cell cultures, modified and transferred back into the same patient.
The expectation for exosomes as drug carriers is rooted in the unique structure of exosomes. Its structure is simple, the outside is a membrane composed of a phospholipid bilayer, and the membrane is rich in protein distribution, and the inside is a cavity, which can be loaded with macromolecules, small molecules and nucleic acids. The cavity is a space we can use for drug delivery. The existence of external proteins is very valuable. On the one hand, it can provide low immunogenicity and great potential for repeatable administration. On the other hand, these proteins can be used for surface modification, loading macromolecules, and improving targeting.

Figure 2 Exosome structure
The medical potential of exosomes mainly includes three major directions (Figure 3):
A. Potential of exosomes in diagnostic prevention: Exosomes extracted from the case microenvironment can be used as biomarkers for the diagnosis of specific diseases and injuries.
B. Medical potential of exosomes: Exosomes are produced by a variety of cells and interact with target cells in various ways to produce medical effects.
C. Drug delivery potential of exosomes: Exosomes can be used to deliver a variety of drugs such as RNA, proteins, and small molecules.

Figure 3 Potential applications of exosomes
3. Uptake And Potential Targeting of Exosomes
Exosomes originate in the late endocytosis and can spread into the intercellular fluid. Exosomes can transport substances through rapid fusion with target cells or receptor-mediated endocytosis. Upon reaching a specific recipient cell, exosome surface molecules bind to membrane receptors, including intercellular adhesion molecules, lymphocyte function associated antigen 1 and TIM1 (TIM4). Finally, the contents of the exosome are released into the cytoplasm, causing changes in the intracellular compartment of the recipient cell. Secreted exosomes may be taken up by target cells through three potential mechanisms (Figure 4).
A. Through simple fusion of cell membranes.
B. Endocytosis.
C. Activation of target cells through specific surface ligands. Some protein components in exosomes may contribute to the formation of protective coats as well as stable vesicle structures and may carry targeting information.
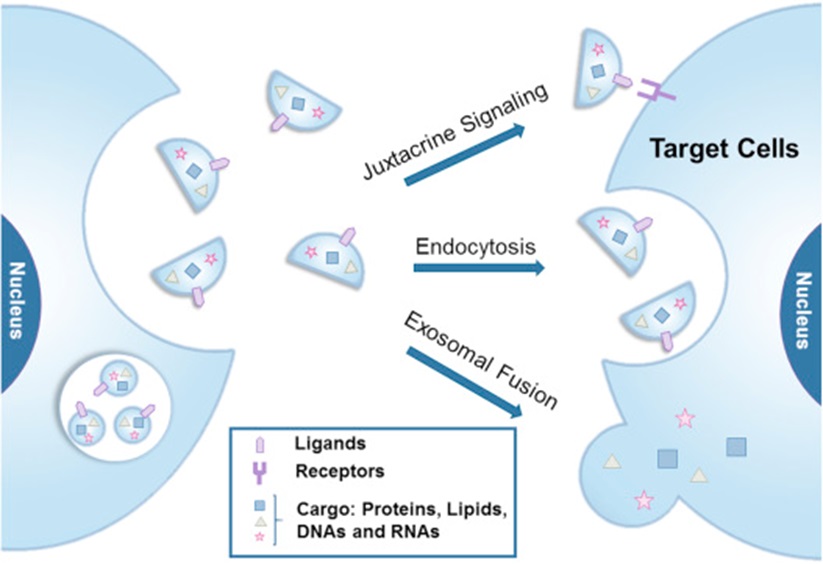
Figure 4 Mechanism of exosome uptake by target cells
(Source: References[2])
4. Comparison of Liposome And Exosome Administration
The main challenges in delivering therapeutic agents to the site of action are off-target toxicity, rapid clearance, and low accumulation and bioavailability in target tissues, cells, or organelles. To circumvent these challenges, a wide range of synthetic delivery vectors (liposomes, lipid nanoparticles, polymer micelles, inorganic nanoparticles, dendrimers, etc.) have been developed over the past few decades, some of which have been clinically approved. Of all the available maps of nanoparticles, the most successful and clinically approved vector on the market to date is liposome. Due to the similarity between liposomes and exosomes, physicochemical properties and drug delivery capabilities of the two will be compared next.
A. Liposomes: Lipid drugs are loaded into the bilayer membrane; ligands can be incorporated to increase tissue targeting specificity; hydrophilic drugs can be loaded in the lumen of liposomes. Onpattro is the first siRNA-loaded lipid nanoparticle approved by the U.S. Food and Drug Administration (FDA) and consists of ionizable lipids, cholesterol, PEGylated lipids, and helper lipids.
B. Exosomes: Proteins, hydrophilic drugs, and nucleic acids (miRNA, siRNA, mRNA, etc.) can be loaded into the lumen of vesicles, while targeting ligands, membrane proteins, and lipophilic drugs can be incorporated into the membrane.

Figure 5 Liposomes and exosomes
Physical Characteristics, Production and Quality Control
Liposomes are structurally similar to exosomes in that they are composed of lipid bilayers. Similarly, exosomes can carry hydrophobic drugs within the lipid membrane bilayer and hydrophilic drugs in the aqueous core. Furthermore, clinically approved liposomes are approximately 100 nm in size, similar to exosomes. In addition, the size of liposomes allows for intravenous administration and extravasation in certain parts of the body after cell uptake.
Despite their similarities, there are many differences between liposomes and Extracellular Vesicles (EVs) as drug delivery vehicles. Compared to exosomes, liposomes for clinical use are composed of a limited number of lipids but have no cellular components such as proteins and genetic material, so they are relatively easy to handle during pharmaceutical quality control and large-scale production.
However, exosomes are rich in sphingomyelin, cholesterol, and lysophospholipids, so exosomes can achieve a higher degree of complexity than mixing individual components in liposomes. In addition, due to the presence of biomolecules in the membrane and core, additional binding pockets may exist in exosomes for drug loading. This requires higher requirements for manufacturing and quality control, and scaling up of exosomes has so far been extremely challenging in terms of production and harvesting.
In Vivo Administration of Exosomes And Liposomes
Nanoparticles (exosomes and liposomes) are rapidly cleared by the mononuclear phagocyte system (MPS). Liposomes represent biodegradable and biocompatible DDS with very versatile high-throughput preparation and drug encapsulation efficiency, allowing lyophilization and surface modification. To reduce immunogenicity and avoid rapid blood clearance of liposomes, polyethylene glycol (PEG) surface coatings are widely used, allowing more accumulation in target tissues. Decorating exosomes with PEG or PEG-conjugated targeting ligands has been proposed as a promising strategy to enhance the drug delivery capacity of exosomes. Another interesting strategy is to select a subset of exosomes containing specific surface proteins such as CD47. This protein acts as a "don't eat me" signal in exosomes and may give them the ability to bypass MPS and exhibit longer circulation times.
Biodistribution
All approved liposomal drugs on the market rely on passive targeting, and only a small percentage of active targeting agents have reached the clinical stage. This is because even when surface ligands are used to target specific receptors on target cells, the accumulation of liposomes is still thought to be determined by a passive extravasation process known as the enhanced permeability and retention (EPR) effect. Through the EPR effect, liposomes with longer circulation times tend to accumulate in tumors or damaged myocardium.
Pharmacokinetics and Pharmacodynamics (PK/PD)
PK/PD, as a simulation system based on the physiological and pharmacological effects of drugs, can provide valuable information for the therapeutic efficacy of drugs. Encapsulation of the drug in the liposome prevents rapid clearance and significantly alters the PK characteristics of the drug compared to the free form. Exosomes may have the potential to reduce MPS-mediated clearance compared to liposomes due to the presence of surface CD47, but more evidence is needed. Due to the challenges of large-scale exosome production and the presence of endogenous exosomes, little information is available on the PK/PD characteristics of exosomes. A comprehensive understanding of the PK/PD properties of exosomes as DDS is critical for exosomes to reach the clinic.
Challenges of Exosomes as Carriers
A key issue in exploring exosomes for clinical applications is the lack of consensus on the best methods to obtain high yields of pure exosomes. This is mainly due to the relatively low number of exosomes released by mammalian cells. Furthermore, the purification of exosomes is cumbersome. There are several methods for isolating exosomes from cell culture supernatants or biological fluids such as milk, urine, plasma, amniotic fluid, saliva, and cerebrospinal fluid (see Table 1). Each of these methods has advantages and disadvantages.

(Source: References[2])
To obtain high yields of pure exosomes, the first route is to expand the source of exosomes. In addition to this, efforts have been made to combine the properties of cells and nanocarriers. In addition, it is very important to be able to enhance the loading capacity and targeting ability of various cargoes without destroying exosomes. Therefore, many researchers have devoted themselves to developing suitable methods to modify exosomes to load drugs or genes.
Conclusion
Exosomes, which have received a lot of attention in the field of biomedicine in recent years, have unique advantages, such as easy loading of multiple molecules, potential for targeting, potential for engineering, low immunogenicity, and suitable for repeated administration. As a new research hotspot, exosomes have become a potentially effective method for disease diagnosis and treatment, and have bright prospects. Of course, exosomes have some limitations of their own. At the present stage, the research on exosomes is not abundant, so the productivity is relatively low, which is also a direction that needs to be improved in this field. Although, the use of exosomes as drug or gene delivery vehicles is still in its infancy. We believe that with the deepening of exosome research, exosome therapy may eventually lead to major breakthroughs in the field of drug or gene delivery.
Polyethylene glycol (PEG) is widely utilized in drug delivery and nanotechnology due to its reported“stealth”properties and biocompatibility. Biopharma PEG has been focusing on the development of a full range of medical applications and technologies for nanocarrier systems (including various types of nanoparticles, liposomes, micelles, etc.), and has accumulated a large number of data models and rich research experience in the construction and optimization of nanocarriers for gene vaccines and protein drugs. We can produce and provide Cholesterol (Plant-Derived), DSPE, classic PEG lipids, such as mPEG-DMG, ALC-0159 and mPEG-DSPE for your LNPs R&D.
References:
[1]. Lee, Vincent H L, and Hamidreza Ghandehari. “Advanced drug delivery: perspectives and prospects. Preface.”Advanced drug delivery reviews vol. 65,1 (2013): 1-2. doi:10.1016/j.addr.2012.12.001
[2]. Exosomes as novel bio-carriersfor gene and drug delivery
Related articles:
[1].New Progress In Lipid Nanoparticles Technology
[2].Lipid Nanoparticles: Key Technology For mRNA Delivery
[3].Lipid Nanoparticles for Drug and Vaccine Delivery
[4].Liposomes vs. Lipid Nanoparticles: Which Is Best for Drug Delivery?
[5].Advantages and Disadvantages of PEGylated Liposomes in Drug Delivery System
[6].Stealth Liposomes (PEGylated Liposomes) as Drug Carrier System for Drug Delivery

