Immune checkpoint inhibitors have become a vital pillar in cancer immunotherapy, with monoclonal antibodies long dominating the PD-1/PD-L1 axis. However, advances in biotechnology are driving the evolution of new therapeutic modalities. One standout example is BMS-986238, an innovative macrocyclic peptide-based PD-L1 inhibitor developed by Bristol Myers Squibb (BMS), poised to reshape the competitive landscape of checkpoint inhibition.
From BMS-986189 to BMS-986238: Next-Generation Design
BMS-986238 builds upon BMS's long-term efforts in the checkpoint inhibitor space. As a next-generation macrocyclic peptide PD-L1 inhibitor, it not only continues the company's strategic focus on this target, but also overcomes key challenges typically associated with peptide therapeutics—namely short half-life and limited oral bioavailability.
The first-generation PD-L1 inhibitor, BMS-986189, demonstrated near-complete peripheral PD-L1 occupancy at low plasma concentrations in healthy volunteers, consistent with its low picomolar binding affinity. However, its short half-life necessitated once-daily dosing (QD) administration, limiting clinical practicality.
To address these limitations, BMS conducted comprehensive molecular optimization, leading to the development of BMS-986238, which features significantly improved pharmacokinetics and strong potential for oral administration.
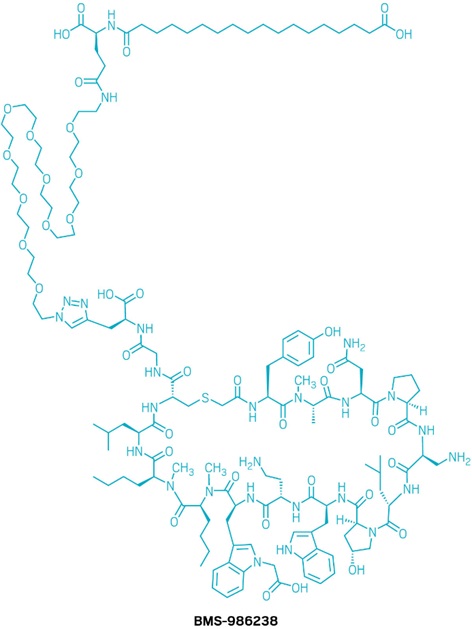
Figure 1. Structure of BMS-986238 [1]
Key Design Strategies Behind BMS-986238
Fatty Acid Acylation
The incorporation of a fatty acid (specifically a C18 fatty di-acid chain) greatly enhances reversible binding to serum albumin, thereby extending circulation time. This strategy, validated by approved GLP-1 receptor agonists like semaglutide and tirzepatide, helps protect the peptide from rapid degradation.
PEG Linker Integration
To ensure the fatty acid does not interfere with PD-L1 binding, a polyethylene glycol (PEG) linker was inserted between the lipid tail and the peptide core. PEG serves two key functions:
- • Acts as a flexible spacer, preserving binding affinity by reducing steric hindrance near the active site.
- • Increases molecular size, lowering renal clearance and slowing metabolism—further extending half-life.
Additional Structural Optimizations
BMS-986238 incorporates a variety of other structural features that support the development of oral peptide therapeutics, including a cyclic peptide backbone, N-methylated amino acids, non-proteinogenic amino acids, and lipidated side chains—all contributing to enhanced stability, permeability, and pharmacokinetic performance.
The result is BMS-986238, a drug boasting exceptional properties: picomolar-level PD-L1 binding affinity, a long half-life (over 19 hours in both rats and cynomolgus monkeys), and promising oral bioavailability. The drug recently completed Phase 1 clinical trials (NCT0668458), establishing a strong foundation for future development.
Differentiated Strategy: Beyond Traditional Antibodies
BMS's public disclosure of the molecular structure of BMS-986238 signals a new phase of development. Unlike conventional monoclonal antibodies, this cyclic peptide introduces a distinct modality, representing a shift toward smaller, more versatile molecules in the highly competitive PD-1/PD-L1 space.
BMS's development strategy focuses on harmonizing the therapeutic window with the exposure profile instead of merely chasing higher absolute bioavailability. Existing data show that BMS‑986189 achieves full PD-L1 occupancy at very low plasma concentrations. If the next‑generation molecule, BMS-986238, retains comparable affinity while providing a significantly longer systemic exposure, it should sustain efficacy at a lower Cmax. This reasoning supports the feasibility of an oral formulation and highlights BMS’s sophisticated approach to PK/PD integration.
Conclusion: The Strategic Value of PEG in Peptide Drug Design
The success of BMS-986238 not only validates the potential of cyclic peptides as PD-L1 inhibitors, but also highlights the critical role of PEG technology in the design of modern peptide therapeutics.
As a specialized PEG manufacturer, we understand the importance of:
- • Selecting the right molecular weight, branching, and functional groups for specific pharmacokinetic outcomes;
- • Offering custom-designed PEG linkers that are essential for translating peptide drugs from the lab to the clinic.
We are committed to supporting global pharmaceutical companies with high-purity, tailor-made PEG derivatives, available from grams to 100 kilograms scale. Whether you’re optimizing peptide half-life, improving oral bioavailability, or fine-tuning drug exposure, our PEG technologies can help bring your molecule closer to clinical success.
References:
[1]12 drug candidates debut in San Diego. Retrieved April 8, 2025, from https://cen.acs.org/acs-news/acs-meeting-news/12-drug-candidates-debut-San/103/web/2025/03
[2] Discovery of BMS-986238, a second-generation macrocyclic peptide inhibitor of programmed death-ligand 1 (PD-L1). https://acs.digitellinc.com/p/s/discovery-of-bms-986238-a-second-generation-macrocyclic-peptide-inhibitor-of-programmed-death-ligand-1-pd-l1-620196

