On August 15, 2025, the U.S. Food and Drug Administration (FDA) has approved Wegovy (semaglutide 2.4 mg) injection to treat metabolic-associated steatohepatitis (MASH) in adults with moderate-to-advanced fibrosis (stages F2–F3). The therapy is indicated for use in combination with a reduced-calorie diet and increased physical activity. [1]
Wegovy® is now the world’s first and only GLP-1 receptor agonist to treat MASH and the and the second treatment ever authorized by the FDA for MASH. The first approved therapy, Resmetirom (Rezdiffra™)—developed by Madrigal Pharmaceuticals—received FDA approval in March 2024. It generated $212.8 million in sales in the second quarter of 2025, with total sales for the first half of the year reaching $350.1 million.
Approval Based on ESSENCE Results
This approval was primarily based on positive results from Part 1 of the Phase III ESSENCE trial. ESSENCE is a large, randomized, double-blind, placebo-controlled study designed to enroll 1,200 adults with MASH and stage F2–F3 liver fibrosis. Participants were randomized 2:1 to receive once-weekly subcutaneous injections of semaglutide 2.4 mg or placebo, in addition to standard care, for a planned treatment duration of 240 weeks.
The approval was supported by liver biopsy–based histological assessments from the first 800 patients after 72 weeks of treatment:
Primary Endpoint 1: Resolution of steatohepatitis with no worsening of liver fibrosis
- ·Semaglutide group: 62.9% achieved the endpoint
- ·Placebo group: 34.3% achieved the endpoint
Primary Endpoint 2: Improvement in liver fibrosis with no worsening of steatohepatitis
- ·Semaglutide group: 36.8% achieved the endpoint
- ·Placebo group: 22.4% achieved the endpoint
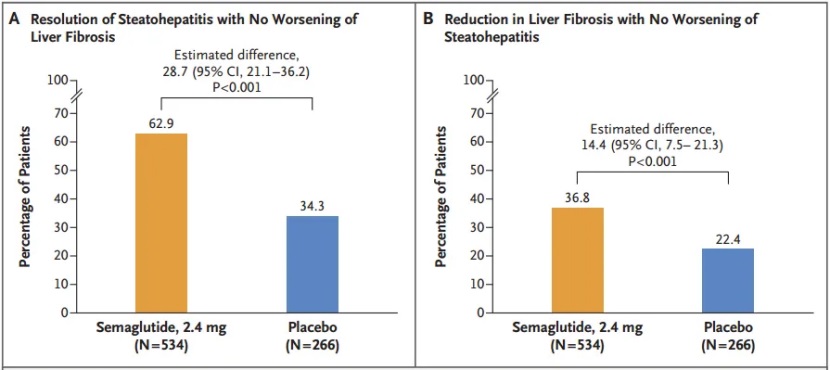
Figure 1. Endpoints of Phase III ESSENCE trial, image source: Novo Nordisk official website
These findings clearly show that semaglutide 2.4 mg not only promotes resolution of steatohepatitis but also significantly improves existing liver fibrosis, providing a powerful therapeutic option to slow or halt MASH progression. Detailed results have been published in the New England Journal of Medicine (NEJM). [2]
The second part of the ESSENCE trial will extend to 240 weeks, focusing on whether semaglutide lowers the risk of liver-related clinical events compared with placebo.1 Findings are expected to read out in 2029.
Other Promising Pathways in MASH
Beyond semaglutide, several other therapeutic approaches are showing strong potential in the treatment of MASH.
Pan-PPAR Agonists
Lanifibranor
Lanifibranor is a pan-PPAR agonist with balanced α, β/δ, and γ activity that modulates key metabolic, inflammatory, and hepatic fibrotic pathways in the pathogenesis of NASH. In the Phase IIb MAESTRO-NASH trial, both the 1200-mg and 800-mg doses of lanifibranor demonstrated superiority over placebo across key endpoints: resolution of NASH without worsening of fibrosis (49% and 39% vs. 22%), improvement of at least one fibrosis stage without worsening of NASH (48% and 34% vs. 29%), and combined resolution of NASH with at least one-stage fibrosis improvement (35% and 25% vs. 9%). A phase 3 trial of lanifibranor in patients with NASH and stage 2–3 fibrosis (NATiV3 trial) is currently ongoing. [3]
FGF21 Analog
Pegozafermin
Pegozafermin is an investigational FGF21 analog engineered with glycoPEGylation technology designed to recapitulate the activity profile and prolong the half-life of the native FGF21 hormone, which may address underlying metabolic issues that drive liver and cardiometabolic diseases, such as MASH and SHTG. In June 2023, The New England Journal of Medicine published a multicenter, randomized controlled trial evaluating Pegozafermin in 219 biopsy-confirmed patients with moderate (F2) or advanced (F3) NASH. After 24 weeks of treatment with either placebo, Pegozafermin 15 mg weekly, Pegozafermin 30 mg weekly, or Pegozafermin 44 mg every two weeks, the proportions of patients achieving fibrosis improvement were 7%, 22%, 26%, and 27%, respectively. The corresponding rates of NASH resolution were 2%, 37%, 23%, and 26%.
The ongoing Phase 3 ENLIGHTEN-Fibrosis trial in non-cirrhotic (F2–F3) MASH and the Phase 3 ENLIGHTEN-Cirrhosis trial in compensated cirrhotic (F4) MASH are actively enrolling patients. Topline histology results from ENLIGHTEN-Fibrosis are anticipated in the first half of 2027, followed by results from ENLIGHTEN-Cirrhosis in 2028.
The Phase 3 ENLIGHTEN-Fibrosis trial in non-cirrhotic (F2-F3) MASH and Phase 3 ENLIGHTEN-Cirrhosis trial in compensated cirrhotic (F4) MASH continue to enroll patients, with topline data from the histology cohorts of these trials expected in the first half of 2027 and in 2028, respectively.
Efruxifermin
Efruxifermin, a bivalent Fc-FGF21 analog developed by Akero Therapeutics, functions as a balanced agonist of FGFR1c, FGFR2c, and FGFR3c through activation of the β-Klotho co-receptor. In adipocytes, FGFR1c activation suppresses lipolysis and enhances adiponectin secretion, thereby reducing hepatic fatty acid influx. Meanwhile, FGFR2c/3c activation in hepatocytes inhibits de novo lipogenesis and limits lipid accumulation.
Recently, The Lancet published long-term data from the Phase II HARMONY trial, highlighting the strong therapeutic potential of efruxifermin. The therapy demonstrated significant anti-fibrotic efficacy: at week 96, 49% of patients in the high-dose group achieved at least a one-stage fibrosis improvement without worsening of MASH. Among patients who underwent biopsy, the response rate rose to 75%, with nearly 30% achieving near-complete disease reversal.
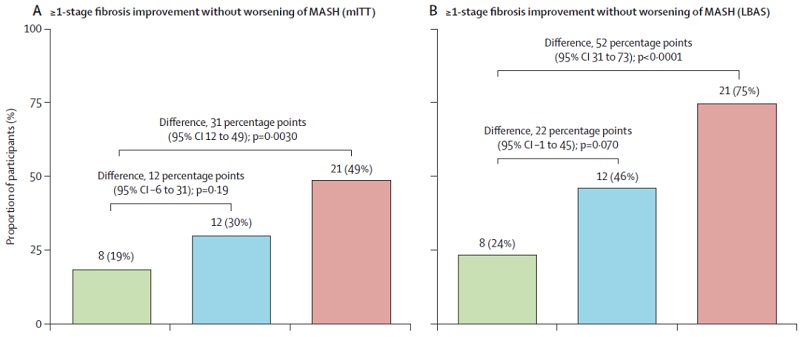
Figure 2. Efruxifermin phase 2b trial [4]
FASN Inhibitor
Denifanstat is a once-daily oral small molecule fatty acid synthase (FASN) inhibitor being developed by Ascletis. By blocking FASN, it prevents the synthesis of palmitate at its source, thereby reducing hepatic steatosis, inflammation, and fibrosis. In a Phase II study reported in 2024 involving 168 patients with NASH, moderate to advanced fibrosis (F2–F3), and NAS ≥4, a significantly greater proportion of patients in the denifanstat group achieved the primary endpoint compared with placebo, with statistically meaningful improvements in fibrosis as well. Denifanstat also reduced liver fat as measured by MRI-PDFF and lowered FAST scores.
The FDA has granted Breakthrough Therapy designation to denifanstat for the treatment of non-cirrhotic NASH with moderate to advanced fibrosis. A Phase III trial is scheduled to begin in the second half of 2025, positioning denifanstat to potentially become the first approved FASN inhibitor for NASH.
Conclusion
The FDA’s approval of Wegovy® (semaglutide 2.4 mg) as the first GLP-1 receptor agonist for MASH represents a landmark milestone in the management of this progressive liver disease. By demonstrating significant efficacy in both fibrosis improvement and steatohepatitis resolution, Wegovy sets a new benchmark for therapeutic innovation.
Meanwhile, a robust pipeline—including pan-PPAR agonists, FGF21 analogs, and FASN inhibitors—continues to advance, with several candidates showing promising efficacy in Phase II and III trials. Together, these developments signal the beginning of a transformative era in MASH treatment, where patients may soon benefit from a diverse portfolio of targeted therapies designed to halt or even reverse disease progression.
References:
[1] FDA Approves Treatment for Serious Liver Disease Known as ‘MASH’ https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-serious-liver-disease-known-mash
[2] Sanyal, AJ ∙ Newsome, PN ∙ Kliers, I ∙ et al., Phase 3 trial of semaglutide in metabolic dysfunction-associated steatohepatitis, N Engl J Med. 2025; 392:2089-2099
[3] Francque, S.M. ∙ Bedossa, P. ∙ Ratziu, V. et al., A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH, N Engl J Med. 2021; 385:1547-1558
[4] Mazen Noureddin, et al., (2025). Safety and efficacy of once-weekly efruxifermin versus placebo in metabolic dysfunction-associated steatohepatitis (HARMONY): 96-week results from a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. The Lancet, DOI: 10.1016/S0140-6736(25)01073-6
[5] Rohit Loomba, M.D., M.H.Sc. et al., Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH, N Engl J Med 2023;389:998-1008, DOI: 10.1056/NEJMoa2304286

