On August 21, 2025, Ionis Pharmaceuticals announced that its RNA-targeted therapy DAWNZERA™ (donidalorsen) has been approved by the U.S. Food and Drug Administration (FDA) for the prevention of hereditary angioedema (HAE) attacks in adults and pediatric patients aged 12 years and older. DAWNZERA™ is the first RNA-targeted therapy worldwide approved for HAE, representing a major breakthrough in this therapeutic area.
Donidalorsen is a ligand-conjugated antisense oligonucleotide (LICA) designed to target and reduce the production of prekallikrein (PKK), a precursor of plasma kallikrein. By lowering PKK levels, donidalorsen prevents excessive bradykinin generation thereby reducing the frequency and severity of attacks in patients with HAE.
The FDA approval was supported by data from the positive results of phase 3 OASIS-HAE trial, a 24-week, randomized, double-blind, placebo-controlled study. The study achieved its primary endpoint, demonstrating that DAWNZERA administered once every four weeks (Q4W) reduced the mean monthly HAE attack rate by 81% compared with placebo over 24 weeks. When assessed from the second dose onward, a key secondary endpoint, the reduction increased to 87%. In addition, DAWNZERA Q4W reduced moderate-to-severe HAE attacks by approximately 90% over 24 weeks, beginning with the second dose.
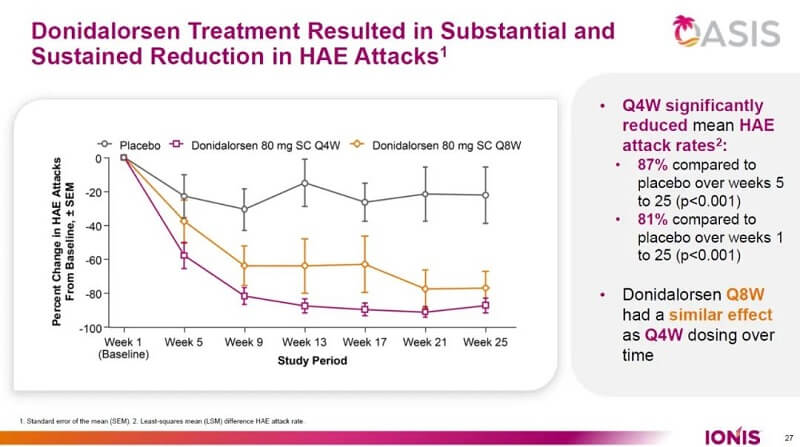
Figure 1. Donidalorsen provides a durable and significant reduction in HAE attack frequency, source: Ionis official website
These efficacy findings were further validated in the Phase 3 OASISplus study, which included both an open-label extension (OLE) cohort and a switch cohort. In the OLE, patients treated with donidalorsen for one year achieved a 94% mean reduction in HAE attack rates from baseline. In the switch cohort, patients transitioning from prior prophylactic therapies to donidalorsen experienced an additional 62% improvement in mean monthly attack rates.
Multiple Promising RNA Therapies
As of the first half of 2025, a total of 24 nucleic acid-based drugs have been approved by FDA, and the field continues to advance rapidly. Beyond donidalorsen, several other RNA-targeted therapies are also showing significant clinical potential.
Plozasiran
Developed by Arrowhead Pharmaceuticals, plozasiran is a GalNac-conjugated siRNA that targets APOC3 mRNA, degrading hepatic APOC3 mRNA transcripts and reducing the production of APOC3 protein with expected reductions in serum triglycerides (TG) and TRL.
On Nov. 18, 2025, Arrowhead announced that the U.S. Food and Drug Administration (FDA) has approved REDEMPLO (plozasiran), a small interfering RNA (siRNA) medicine, as an adjunct to diet to reduce triglycerides in adults with familial chylomicronemia syndrome (FCS). The drug’s New Drug Application (NDA) has been accepted by the FDA for the treatment of familial chylomicronemia syndrome (FCS), with a Prescription Drug User Fee Act (PDUFA) date set for November 18, 2025. If approved, plozasiran would become the first RNAi therapy for FCS. Results from the pivotal Phase 3 PALISADE trial showed that after 10 months of treatment, patients experienced a median triglyceride reduction of 80% from baseline and an 83% reduction in the risk of developing acute pancreatitis.
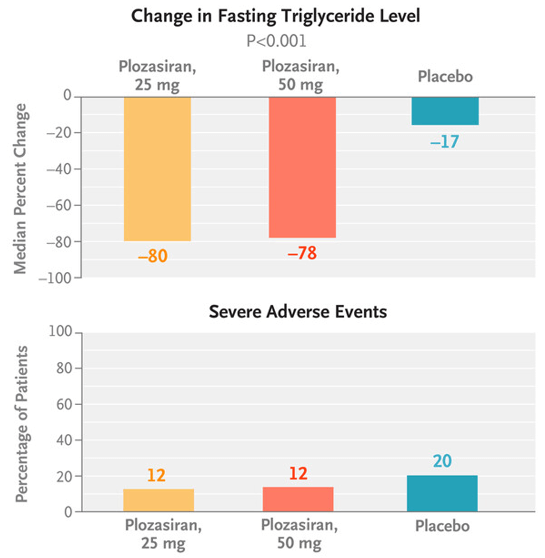
▲ Plozasiran significantly reduced triglyceride levels while demonstrating a favorable safety profile, Image source: [2]
Pelacarsen
Co-developed by Ionis and Novartis, pelacarsen is an antisense oligonucleotide (ASO) designed to lower lipoprotein(a) [Lp(a)] levels in patients at high risk of cardiovascular events. The Phase 3 Lp(a) HORIZON trial is currently ongoing, with primary endpoint of the time to the first major adverse cardiovascular event (MACE). Although completion was originally expected in 2025, the timeline has been extended to February 2026 to allow enough events to be recorded. Phase 2 data demonstrated that pelacarsen can lower Lp(a) levels by approximately 80%, with no significant safety signals, positioning it as a potential first-in-class therapy pending CVOT results. [3]
Ulefnersen
Ulefnersen, developed by Shneider in collaboration with Ionis Pharmaceuticals, is an antisense oligonucleotide (ASO) therapy for the treatment of patients with amyotrophic lateral sclerosis (ALS) caused by mutation of the fused in sarcoma (FUS) gene. Ulefnersen is now being evaluated in an international, multicenter Phase 1-3 trial for patients with FUS-ALS. If development and then regulatory reviews proceed successfully, ulefnersen could become the first treatment for FUS-ALS. [4]
Together, these developments highlight the expanding therapeutic frontier of RNA-targeted medicines across genetic disorders, metabolic conditions, cardiovascular disease, and neurodegenerative disorders—offering new treatment possibilities for conditions with limited or no effective interventions.
References:
[1] https://ir.ionis.com/news-releases/news-release-details/dawnzeratm-donidalorsen-approved-us-first-and-only-rna-targeted DAWNZERA™ (donidalorsen) approved in the U.S. as first and only RNA-targeted prophylactic treatment for hereditary angioedema
[2] https://ir.arrowheadpharma.com/news-releases/news-release-details/arrowhead-pharmaceuticals-announces-acceptance-new-drug Arrowhead Pharmaceuticals Announces Acceptance of New Drug Application by U.S. FDA of Plozasiran for the Treatment of Familial Chylomicronemia Syndrome
[3] https://www.pharmacytimes.com/view/nla-2025-novel-therapeutic-approaches-show-promise-for-targeted-reduction-of-lp-a-in-patients-with-ascvd NLA 2025: Novel Therapeutic Approaches Show Promise for Targeted Reduction of Lp(a) in Patients With ASCVD
[4] https://neurology.ionis.com/sites/default/files/2024-10/Fused%20in%20Sarcoma%20Amyotrophic%20Lateral%20Sclerosis%20Clinical%20Trial%20Handout.pdf
Related Articles:
Small Nucleic Acid Drugs Expected to Be Approved by 2025
Nucleic Acid Therapeutics: Approvals and Potential Blockbusters
Aptamer Therapeutics: Current Status
siRNA Therapeutics: Current Status & Delivery System

