Fibroblast growth factor 21 (FGF21), a hormone that beneficially regulates glucose and lipid metabolism and contributes to energy balance, was once constrained by translational limitations and received limited industry attention. Recent high-value transactions have shifted this landscape. In 2025, global deal value related to FGF21 analogs has surpassed $9.6 billion, underscoring renewed confidence in this pathway.
This resurgence is driven by deeper insights into the FGFR1/KLB signaling pathways and the maturation of second-generation engineering strategies. As a result, FGF21 analogs are now regarded as one of the most promising therapeutic classes for MASH (metabolic dysfunction-associated steatohepatitis).
As a supplier of PEG derivatives and PEGylation technologies, Biopharma PEG has closely observed the pivotal role of PEG in enabling the early development and optimization of this therapeutic class.
FGF21: Major M&A Deals in 2025
GSK × Boston Pharmaceuticals – Efimosfermin alfa
In May 2025, GSK acquired Boston Pharmaceuticals' lead asset, efimosfermin alfa, for $2 billion (an upfront payment of $1.2 billion and up to $800 million in success-based milestone payments). Efimosfermin alfa is a long-acting, once-monthly FGF21 analogue in clinical development for the treatment of MASH, including cirrhosis.
Roche × 89bio – Pegozafermin
In September 2025, Roche acquired 89bio in a $3.5 billion deal centered on the FGF21 analog pegozafermin being developed for MASH.
Novo Nordisk × Akero – Efruxifermin
In October 2025, Novo Nordisk acquired Akero for $5.2 billion, adding the lead asset Efruxifermin and reinforcing its position in metabolic therapeutics, especially within GLP-1–based combination strategies.
FGF21R: Mechanism of Action
FGF21 is primarily secreted from the liver and adipose tissue, which regulates energy homeostasis and has pleiotropic metabolic effects. FGF21 can bind and activate 3 FGFR isoforms (1c, 2c and 3c) in the presence of the co-receptor βKlotho (KLB) in vitro. As a non-canonical fibroblast growth factor, FGF21 functions as an endocrine hormone that signals to distinct targets throughout the body.
- ● Liver: FGF21 blocks hepatic lipid influx and accumulation and improves insulin sensitivity, helping alleviate hepatocellular injury and inflammation at the source.
- ● Adipose tissue: FGF21 promotes glucose utilization and increases energy expenditure
- ● Central nervous system: FGF21 modulates appetite and reduces cravings for sugar and alcohol, showing complementary effects with GLP-1–based therapies.
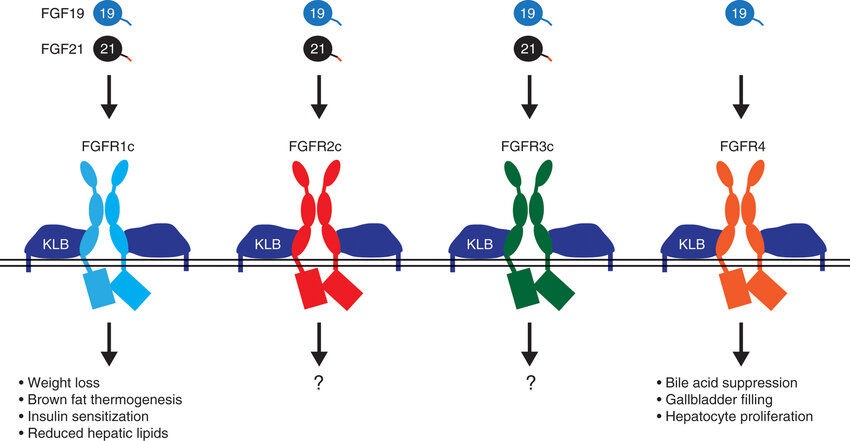
Figure 1. FGF19 and FGF21 receptor specificity and function. Source: reference [1]
Compared with treatments that target a single metabolic parameter, FGF21 offers a unique, system-level approach through one molecule acting across multiple metabolic pathways—providing a promising new direction for comprehensive management of metabolic diseases.
FGF21: Clinical Progress
First Generation: Pegbelfermin (BMS‐986036), PEGylated FGF21
The native FGF21 protein has poor pharmacokinetic properties, including a short half‐life (ranging from 0.5 to 2 h), making engineering optimization essential for therapeutic use. Early programs relied heavily on PEGylation to prolong circulation, and BMS’s Pegbelfermin (BMS-986036) was developed using this strategy.
Despite promising early signals, Pegbelfermin did not meet the primary endpoints in two Phase IIb studies (FALCON 1 and FALCON 2). In November 2021, BMS announced the termination of the program.
Second-Generation FGF21 Analogs: Improved Binding, Stability, and Exposure
After early programs encountered challenges, developers moved beyond simple half-life extension and focused on redesigning the FGF21 molecule itself. The aim was to achieve longer exposure while strengthening receptor affinity, enhancing structural stability, and improving overall pharmacology. Several engineering strategies have since emerged, with three leading molecules representing the most established approaches.
Pegozafermin: Glycopegylated Analog
Pegozafermin continues to use PEG as its key modification but applies glycoPEGylation technology with site-specific mutations designed to recapitulate the activity profile and prolong the half-life (55 to 100 hours) of the native FGF21 hormone.
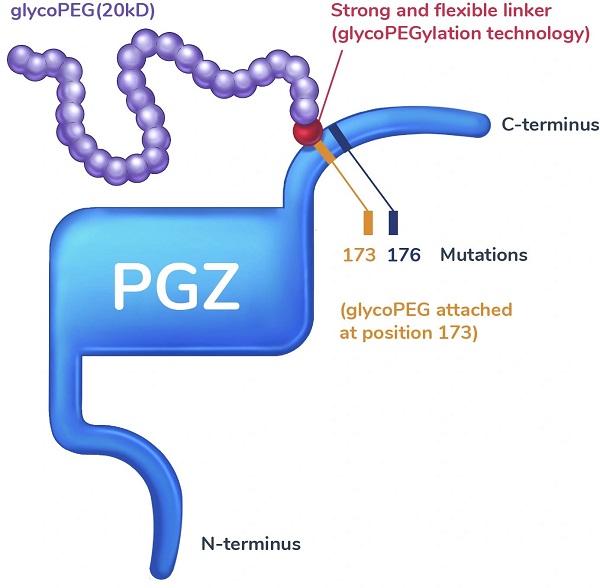
Figure 2. Structure of Pegozafermin, source: https://89bio.com/
In the Phase IIb ENLIVEN trial in patients with F2–F3 MASH, pegozafermin showed clear benefits over 24 weeks at every-2-week 44-mg dose: [2]
- ● 27% achieved at least a one-stage improvement in fibrosis vs 7% with placebo (p=0.008)
- ● 26% achieved MASH resolution vs 2% with placebo (p=0.0005)
These improvements were sustained through Week 48 and were also seen in patients on background GLP-1 therapy.
Pegozafermin is being studied in the Phase 3 ENLIGHTEN trial program for MASH and is being studied in the Phase 3 ENTRUST trial for SHTG.
Efruxifermin: Bivalent Fc–FGF21 Analogue
Efruxifermin is a bivalent Fc-FGF21 candidate drug that has shown promising results in preclinical and clinical trials for MASH and may be approved in the future. Unlike monovalent analogs of FGF21, one molecule of EFX comprises two molecules of an FGF21 variant, FGF21[L98R, P171G ,A180E] (RGE).
This bivalent structure would result in greater affinity for FGF21’s receptors on the cell surface compared to monovalent FGF21 analogs, potentially leading to more potent, durable, and effective agonism of FGF21’s target receptors in vivo.
In HARMONY (NCT04767529), efruxifermin achieved statistically robust improvements across steatohepatitis and fibrosis endpoints. [4]
- ● At Week 96 (mITT), ≥1-stage fibrosis improvement without MASH worsening occurred in 19% (placebo), 30% (28 mg), and 49% (50 mg).
- ● Among participants with available Week-96 biopsies, response rates were 24% (placebo), 46% (28 mg), and 75% (50 mg).
Phase 3 SYNCHRONY programs, including SYNCHRONY Histology, SYNCHRONY Outcomes and SYNCHRONY Real-World, are now underway evaluating Efruxifermin for the treatment of pre-cirrhotic (F2-F3) MASH and compensated cirrhosis (F4) due to MASH.
Efimosfermin alfa: Long-acting FGF21 Analogue
Efimosfermin is an investigational, once-monthly subcutaneous injection of a long-acting variant of FGF21 that is designed to regulate key metabolic pathways to decrease liver fat, ameliorate liver inflammation, and reverse liver fibrosis in patients with MASH.
In a Phase 2 trial in patients with F2–F3 MASH, monthly 300 mg efimosfermin achieved statistically meaningful improvements at Week 24: [5]
- ● 45.2% achieved ≥1-stage fibrosis improvement vs 20.6% with placebo (p=0.038).
- ● 67.7% achieved MASH resolution vs 29.4% with placebo (p<0.01).
These findings highlight how advances in protein engineering—such as Fc-fusion design and site-specific PEGylation—can overcome the limitations of first-generation molecules and unlock the full therapeutic potential of the FGF21 pathway.
With extensive expertise in PEG derivatives and precision PEGylation, Biopharma PEG supports the rational design and optimization of next-generation biologics in this rapidly evolving field.
Conclusion
The resurgence of the FGF21 field showcases how advanced protein engineering can translate strong biology into effective therapies. From the setbacks of Pegbelfermin to the success of next-generation molecules, rational drug design proves decisive. With their anti-fibrotic potential, FGF21 analogues are poised to play a central role in the growing MASH and metabolic disease market, complementing existing therapies and reshaping the treatment landscape.
References:
[1] Sonoda J, Chen MZ, Baruch A. FGF21-receptor agonists: an emerging therapeutic class for obesity-related diseases. Horm Mol Biol Clin Investig. 2017 May 19;30(2):/j/hmbci.2017.30.issue-2/hmbci-2017-0002/hmbci-2017-0002.xml. doi: 10.1515/hmbci-2017-0002. PMID: 28525362.
[2] Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH https://www.nejm.org/doi/full/10.1056/NEJMoa2304286
[3] https://akerotx.com/wp-content/uploads/2023/03/Akero_Efruxifermin_Keystone_Pivalent_Poster.pdf
[4] Safety and efficacy of once-weekly efruxifermin versus placebo in metabolic dysfunction-associated steatohepatitis (HARMONY): 96-week results from a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial, Noureddin, Mazen et al., The Lancet, Volume 406, Issue 10504, 719 - 730
[5] https://www.clinicaltrialvanguard.com/news/boston-pharmaceuticals-positive-phase-2-nash-data-at-aasld-2024/

