On October 26, 2025, Novartis announced its $12 billion acquisition of Avidity Biosciences, a pioneer in antibody–oligonucleotide conjugate (AOC™) technology. The acquisition will bring Avidit’s late-stage neuroscience programs into Novartis, including three potential first-in-class therapies for genetic neuromuscular diseases: delpacibart zotadirsen (del-zota) for Duchenne muscular dystrophy (DMD); delpacibart etedesiran (del-desiran) for myotonic dystrophy type 1 (DM1); and delpacibart braxlosiran (del-brax) for facioscapulohumeral muscular dystrophy (FSHD).
For Novartis, this isn't just an acquisition — it’s a declaration of intent. While most pharma giants are pouring billions into antibody-drug conjugates (ADCs), Novartis is charting a different course, betting big on AOCs, a new class of targeted therapies that combine the precision of antibodies with the gene-silencing power of RNA medicines.
Introduction of Antibody-Oligonucleotide Conjugates (AOCs)
AOCs are hybrid molecules that combine the specificity of monoclonal antibodies with the precision of therapeutic oligonucleotides (a siRNA or phosphonodiamidite morpholino oligomer [PMO]). This dual functionality allows for modulation of gene expression while maintaining spatial specificity, offering significant advantages in oncology, rare genetic diseases, and neurological disorders.
In rare genetic diseases, where small patient populations and complex pathologies often hinder drug development, AOCs bring new therapeutic possibilities. Their high specificity and precise targeting enable correction of disease-causing mutations at the genetic level, paving the way for personalized treatments for conditions once considered untreatable.
In oncology, AOCs are emerging as a promising new approach. Tumor cells are highly heterogeneous, making it difficult for conventional drugs to achieve precise targeting. By leveraging antibody-mediated delivery, AOCs transport therapeutic oligonucleotides directly into tumor cells, allowing for gene-level regulation that suppresses tumor growth, induces apoptosis, and minimizes damage to healthy tissue. This provides a novel and more selective strategy for cancer treatment.
AOC Pipelines of Avidity
Avidity Biosciences has expanded its AOC pipelines beyond neuromuscular disorders into the fields of cardiology and immunology, establishing one of the most advanced and diversified AOC portfolios in the world. The company currently has eight major programs, including three in registration-stage studies—Del-zota (DMD Exon 44), Del-desiran (DMPK), and Del-brax (DUX4).
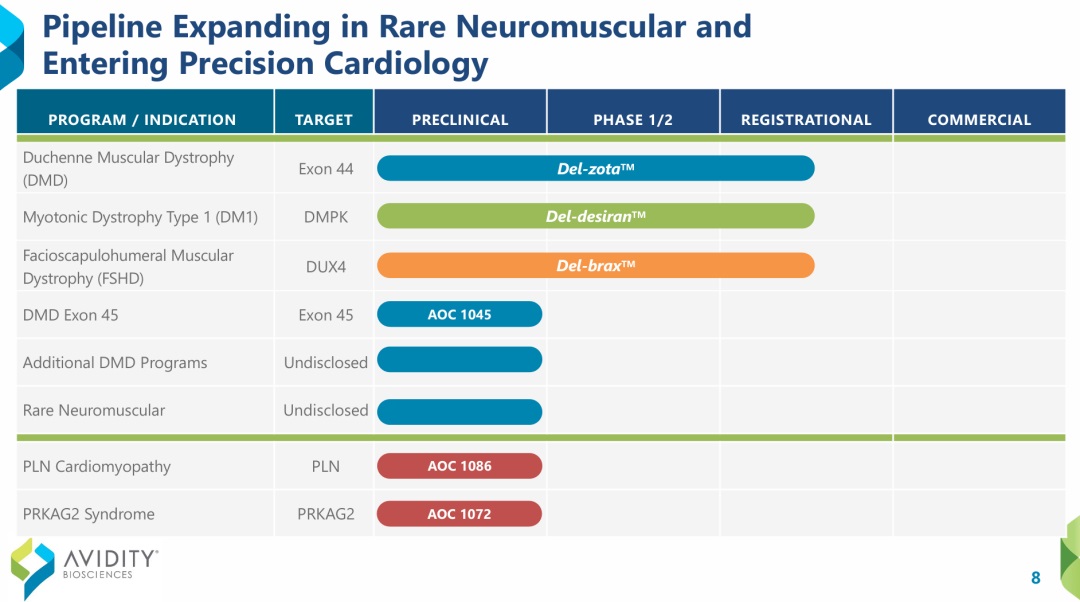
Figure 1. Pipelines of Avidity, source: https://www.aviditybiosciences.com/pipeline/pipeline-overview
Del-zota is an investigational therapy for Duchenne muscular dystrophy (DMD), a monogenic, X-linked, recessive disease that primarily affects males. It is designed to deliver PMOs to skeletal muscle and heart tissue to specifically skip exon 44 of the dystrophin gene to enable dystrophin production in people living with DMD with mutations amenable to exon 44 skipping (DMD44). Results from EXPLORE44 (NCT05670730) show that trial participants on del-zota experienced significant increases of approximately 25% of normal dystrophin production and restored total dystrophin up to 58% normal. Avidity remains on track to submit a biologics license application (BLA) for accelerated approval at the end of 2025. [2]
Del-desiran consists of a proprietary mAb that binds to the transferrin receptor 1 (TfR1) conjugated with a siRNA targeting DMPK mRNA. It is designed to address the root cause of DM1 by reducing levels of a disease-related mRNA called DMPK in skeletal, cardiac, and smooth muscle. Del-desiran has received Breakthrough Therapy, Orphan Drug and Fast Track designations by the U.S. Food and Drug Administration (FDA) and Orphan designation by the European Medicines Agency (EMA). On July 28, 2025, Avidity announced that enrollment for its global Phase III study, HARBOR, had been completed, with top-line results expected in Q2 2026. [3]
Del-brax is the first investigational therapy designed to treat the underlying cause of FSHD by directly targeting the disease-causing gene, double homeobox 4 (DUX4). It is comprised of a DUX4-targeting siRNA conjugated to a humanized anti-TfR1 antibody to facilitate delivery to muscle tissue. Preclinical and early clinical data demonstrate promising efficacy, safety, and tolerability. The FDA and EMA have both granted orphan drug designation for del-brax, and the FDA has granted del-brax Fast Track designation. Avidity plans to initiate a registrational cohort to further validate its clinical benefits.
Beyond its core neuromuscular programs, Avidity is also advancing AOC 1045 and additional candidates for DMD and rare neuromuscular diseases, alongside two early-stage cardiovascular programs—AOC 1086 for PLN-related cardiomyopathy and AOC 1072 for PRKAG2 syndrome.
Global clinical trial pipelines for AOCs
Beyond Avidity Biosciences, several companies — including Dyne Therapeutics and Tallac Therapeutics — have also made notable advances in the development of AOCs.
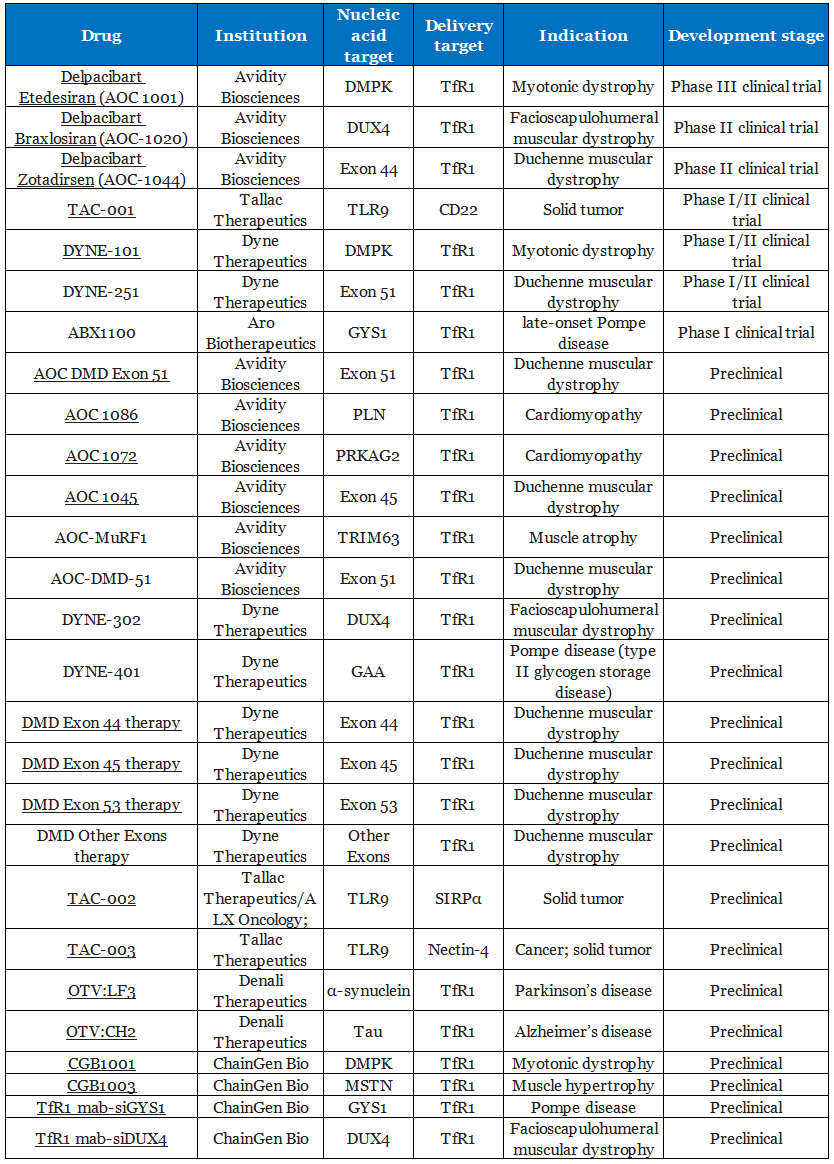
Table 1. Global clinical trial pipelines for AOCs [1]
Dyne Therapeutics
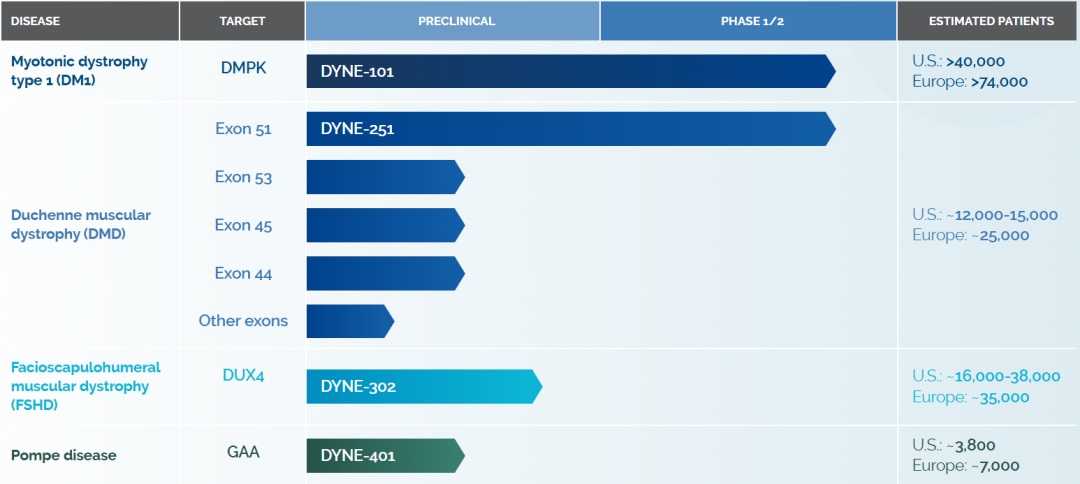
Figure 2. Pipelines of Dyne Therapeutics, source: https://www.dyne-tx.com/pipeline/
DYNE-101 is an investigational therapy currently being studied in the global Phase 1/2 ACHIEVE trial for patients with DM1. It consists of an ASO conjugated to a fragment antibody (Fab) that binds to the TfR1, allowing efficient delivery to muscle and central nervous system tissues. The therapy aims to improve muscle function by reducing toxic DMPK RNA and correcting the spliceopathy underlying the disease. DYNE-101 has received Orphan Drug, Fast Track, and Breakthrough Therapy designations from the U.S. FDA, as well as Orphan Drug designation from the EMA.[4]
DYNE-251 is the company’s next most advanced program. It targets people living with DMD who are amenable to exon 51 skipping. In the Phase I/II DELIVER trial, announced in March 2025, DYNE-251 showed unprecedented efficacy — at a 20 mg/kg dose, treated patients achieved a mean absolute dystrophin expression of 8.72% of normal, a promising result for this difficult-to-treat condition. Dyne plans to submit a Biologics License Application (BLA) to the FDA in early 2026 for accelerated approval.
Tallac Therapeutics
TAC-001 is an investigational, systemically delivered, TRAAC molecule comprised of a potent TLR9 Agonist conjugated to a CD22 antibody, designed to selectively activate B cells to drive an anti-tumor immune response. TAC-001 is being developed for the treatment of solid tumors and is currently in a Phase 1/2 Study in cancer patients (NCT05399654). Emerging clinical data demonstrating tolerability, pharmacodynamic activity and preliminary clinical activity of single agent TAC-001 were observed. [6]
Conslusion
Although AOC development is progressing rapidly, the field remains in its early stages and still faces key challenges. Technical hurdles in conjugation and delivery, the complexity of clinical translation, and the need for rigorous quality control all present barriers to large-scale application. Continued innovation and collaboration between academia and industry will be essential to unlock the full therapeutic potential of antibody-oligonucleotide conjugates.
References:
[1] Li M, An H, Zhang J, Li W, Yu C, Wang L. Advances in the pharmaceutical development of antibody-oligonucleotide conjugates. Eur J Pharm Sci. 2025 Sep 27;215:107292. doi: 10.1016/j.ejps.2025.107292. Epub ahead of print. PMID: 41022318.
[2] https://www.neurologylive.com/view/del-zota-reverses-duchenne-disease-progression-1-year-trial-update
[3] https://www.aviditybiosciences.com/pipeline/dm1
[4] https://www.dyne-tx.com/dyne-101-for-dm1/
[5] https://investors.dyne-tx.com/news-releases/news-release-details/dyne-therapeutics-announces-new-long-term-clinical-data-phase-12
[6] https://www.tallactherapeutics.com/news/tallac-therapeutics-presents-new-preclinical-data-on-tac-001-in-combination-with-cancer-vaccines-at-the-2024-annual-meeting-of-the-american-association-for-cancer-research-aacr/

