Chimeric antigen receptor (CAR)-T cell therapy has revolutionized targeted immunotherapy. The generation of CAR T-cells begins by isolating cells from the patient’s blood, followed by ex vivo activation, expansion and genetic modification to express the CAR receptor. Finally, the CAR-T cells are infused back into the patient. While this approach has shown remarkable success in hematological malignancies, it faces major limitations: manufacturing complexity, high production costs, significant patient-to-patient variability, and limited efficacy against solid tumors.
To break the limitations of ex vivo CAR-T cells, a novel approach has been established to generate CAR-T cells directly in vivo. Unlike the traditional ex vivo approach, in vivo CAR-T therapy selectively transfers CAR genes into the patient’s T cells in the body through targeted delivery systems. This approach bypasses apheresis and ex vivo GMP cell manufacturing.
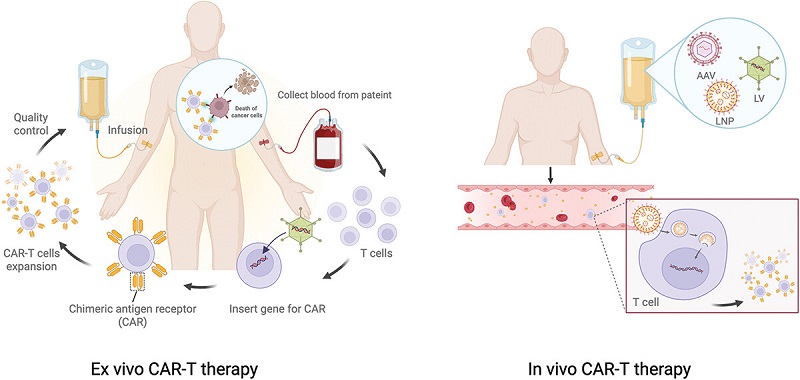
Figure 1. Ex vivo CAR-T and in Vivo CAR-T, source: reference [1]
In vivo CAR-T offers several potential advantages:
l Off-the-shelf accessibility: In vivo CAR-T could be administered directly without the need for complex laboratory infrastructure and personalized manufacturing.
l Reduced cost and time: Bypassing ex vivo manufacturing may significantly shorten preparation time and lower overall costs.
l Repeat dosing and wider accessibility: Enables redosing when needed and opens the door to broader patient access, especially in low-resource settings.
l Improved safety: Minimizes long-term risks associated with permanently modified T cells, offering better safety control.
The Role of Lipid Nanoparticles (LNPs) In In-vivo CAR-T
Despite encouraging progress, in vivo CAR-T therapy still faces technical challenges — particularly the efficient delivery of CAR transgenes to targeted T cells within the body. To address this, both viral vectors (such as lentivirus, retrovirus, and AAV) and non-viral nanocarriers (such as lipid nanoparticles, LNPs) are being explored and utilised for in vivo delivery of CAR constructs.
LNPs are nanoscale, non-viral carriers capable of encapsulating nucleic acids such as mRNA, protecting them from degradation and enabling efficient intracellular delivery. Due to the unique physicochemical features and biocompatibility, LNPs have become an essential approach for in vivo delivery of CAR constructs to T cells.
LNP-mediated mRNA delivery allows transient CAR expression in T cells, minimizing long-term risks such as cytokine release syndrome (CRS). Compared with viral vectors, LNPs-based delivery platforms for in vivo CAR-T cells overcome several problems, including limited vector capacity, insertional mutagenesis, and viral immunogenicity.
Capstan Therapeutics
Capstan Therapeutics, Inc. Is a clinical-stage biotechnology company dedicated to advancing in vivo reprogramming of cells through RNA delivery using targeted lipid nanoparticles (tLNP), which composed of LNPs conjugated with a recombinant protein binder, such as a monoclonal antibody.
Its lead candidate, CPTX2309, is an in vivo anti-CD19 CAR-T therapy that entered a Phase I clinical trial in June 2025 for the treatment of B cell-mediated autoimmune disorders. CPTX2309 employs a tLNP-based delivery approach to delivers an anti-CD19 CAR mRNA preferentially to CD8-expressing cytotoxic T cells, driving CAR expression and targeted elimination of pathogenic B cells. [2]
Technically, Capstan integrates maleimide-modified PEG-lipids into the LNP formulation during preparation. After LNP assembly, antibodies containing thiol groups are conjugated to these maleimide groups through incubation, resulting in antibody-modified, targeted tLNPs. This design allows the nanoparticles to selectively recognize and deliver their RNA payload to specific T cells, enhancing transfection efficiency and therapeutic precision. [4]
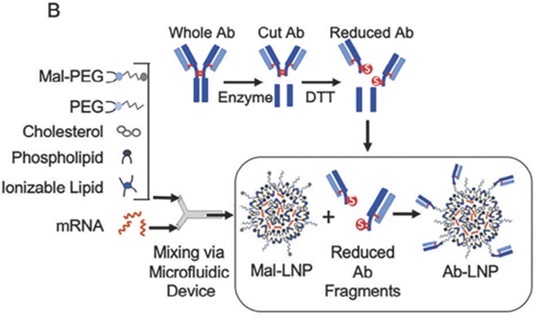
Figure 2. Structure of CPTX2309, source: reference [4]
On June 30, 2025, AbbVie announced a $2.1 billion acquisition of Capstan. The deal includes the clinical-stage program CPTX2309 and Capstan’s proprietary tLNP platform, designed for efficient in vivo RNA delivery and engineering of targeted cell populations.
Myeloid Therapeutics
Myeloid Therapeutics, Inc. is a clinical-stage immunology company pioneering RNA-based therapies to fight cancer. Its proprietary ATAK™ CAR receptor platform reprograms myeloid cells in vivo using mRNA-LNP technology, triggering targeted tumor killing and broad anti-tumor immune responses.
MT-302 is the Myeloid's first in vivo CAR clinical program. It uses synthetic mRNA-LNPs to reprogram circulating immune cells in vivo to express a TROP2-targeted CAR. Preclinical studies show MT-302 achieves robust myeloid cell expression and a favorable safety profile in rodents and non-human primates. Unlike other therapies, MT-302 may trigger a full immune response by presenting tumor neoantigens to stimulate T cells.
MT-303,Myeloid’s second in vivo CAR clinical program, is a first-in-class GPC3-FcA-LNP therapy targeting glypican-3 (GPC3), highly expressed in most human hepatocellular carcinomas (HCCs) and limited in healthy tissues. Like MT-302, MT-303 has shown strong preclinical results, including robust expression in myeloid cells and a favorable safety profile in rodents and non-human primates. MT-303 also has the potential to elicit a complete immune response by presenting tumor neoantigens to T cells.
NanoCell
NanoCell’s NCtx is the first lipid nanoparticle–based system capable of delivering DNA to T cells and generating CAR-T cells in vivo. The NCtx platform uses single-targeted nanoparticles (tLNPs) to co-deliver CAR-encoding DNA and SB100x transposase mRNA, enabling stable integration of the CAR gene and long-lasting expression. This approach produces durable CAR-T cells with potent anti-tumor activity. [5]
Using a CD7/CD3 dual-targeting strategy, NCtx achieves T cell–specific delivery while activating the cells, significantly enhancing DNA uptake efficiency.
In a mouse model of B-cell leukemia, a single intravenous injection of NCtx successfully induced CAR-T cell generation in vivo. This single dose led to stable CAR expression, and the resulting CAR-T cells distributed widely in blood, spleen, and bone marrow. The therapy effectively eliminated tumors and extended survival, remaining active even at low doses. These results provide strong preclinical evidence supporting future clinical translation.
Conclusion
Although in vivo CAR-T therapies are still in early research and clinical development, and face challenges such as target specificity, delivery efficiency, and safety concerns (e.g., cytokine release syndrome), they represent a major next-generation direction in immunotherapy. This approach holds significant potential not only for treating hematologic malignancies but also for addressing solid tumors and other diseases, including autoimmune disorders and rare conditions.
Biopharma PEG is a leading worldwide PEG supplier that dedicated to manufacturing and supplying kilogram scale manufacture of PEG derivatives in both GMP and non-GMP grades, including monodispersed PEGs, polydispersed PEGs and multi-arm PEGs, etc.
References:
[1] Huang Y, Cao R, Wang S, Chen X, Ping Y, Zhang Y. In vivo CAR-T cell therapy: New breakthroughs for cell-based tumor immunotherapy. Hum Vaccin Immunother. 2025 Dec;21(1):2558403. doi: 10.1080/21645515.2025.2558403. Epub 2025 Sep 11. PMID: 40932272; PMCID: PMC12427527.
[2] Bui, T. A., Mei, H., Sang, R., Ortega, D. G., & Deng, W. (2024). Advancements and challenges in developing in vivo CAR T cell therapies for cancer treatment. EBioMedicine, 106, 105266. https://doi.org/10.1016/j.ebiom.2024.105266
[3] https://news.abbvie.com/2025-06-30-AbbVie-to-Acquire-Capstan-Therapeutics,-Further-Strengthening-Commitment-to-Transforming-Patient-Care-in-Immunology
[4] Billingsley MM, Gong N, Mukalel AJ, Thatte AS, El-Mayta R, Patel SK, Metzloff AE, Swingle KL, Han X, Xue L, Hamilton AG, Safford HC, Alameh MG, Papp TE, Parhiz H, Weissman D, Mitchell MJ. In Vivo mRNA CAR T Cell Engineering via Targeted Ionizable Lipid Nanoparticles with Extrahepatic Tropism. Small. 2024 Mar;20(11):e2304378. doi: 10.1002/smll.202304378. Epub 2023 Dec 10. PMID: 38072809.
[5] Bimbo, J. F., van Diest, E., Murphy, D. E., Ashoti, A., Evers, M. J. W., Narayanavari, S. A., Vaz, D. P., Rijssemus, H., Zotou, C., Saber, N., Lei, Z., Mayrhofer, P., Geerlings, M., Schiffelers, R., & Lubelski, J. (2025). T cell-specific non-viral DNA delivery and in vivo CAR-T generation using targeted lipid nanoparticles. Journal for immunotherapy of cancer, 13(7), e011759. https://doi.org/10.1136/jitc-2025-011759
Related Articles:
Advances in Antibody-Targeted Lipid Nanoparticles (Ab-LNPs) and Their Emerging Therapeutic Applications
Application of mRNA-Lipid Nanoparticles (LNPs) In Cancer
Nucleic Acid Therapeutics: Approvals and Potential Blockbusters
The Applications and Challenges of Lipid Nanoparticles
The Role of Four Lipid Components Of LNPs

