Antibody-drug conjugates (ADCs) have revolutionized cancer treatment by delivering cytotoxic drugs selectively to cancer cells that express the target antigen while minimizing exposure to healthy cells. However, the limited diversity of payload types and the emergence of drug resistance have prompted researchers to seek new therapeutic mechanisms. In recent years, Molecular Glue-Antibody Conjugates (MACs) have emerged as a promising concept that combines the precise targeting capability of antibodies with the catalytic, event-driven nature of molecular glues, representing a groundbreaking advance in this field.
Development of ADC and Targeted Protein Degradation (TPD)
ADCs overcome the limitations of traditional chemotherapy, providing higher efficacy and fewer toxicities of anti-tumor biopharmaceuticals. To date, 19 ADCs have been approved for clinical use. Despite their therapeutic benefits, the development and application of ADCs face significant challenges, including limited payload diversity, as most ADCs rely on microtubule inhibitors or DNA-damaging agents, and drug resistance, which reduces long-term efficacy and drives the search for new payload mechanisms.
At the same time, Targeted Protein Degradation (TPD) has rapidly emerged as an innovative therapeutic strategy. Unlike traditional small-molecule inhibitors that chiefly function by blocking the catalytic activity of a druggable protein via an occupancy-driven pharmacology, TPD uses the cell’s ubiquitin-proteasome system (UPS) to remove the disease-causing protein completely.
Within the TPD field, two major classes of degraders have been developed:
- l PROTACs (Proteolysis Targeting Chimeras): comprising an E3-recruiting ligand, a POI-targeting warhead, and a flexible linker linking the two ligands. PROTACs are heterobifunctional degraders that simultaneously interact with the E3 ligase and the POI.
- l Molecular Glue Degraders (MGDs): monofunctional small molecules that induce the interaction between a ubiquitin ligase and a POI, leading to POI ubiquitination and subsequent degradation.
Compared with PROTACs, molecular glues are generally smaller in molecular weight and exhibit better pharmacokinetic properties, including higher membrane permeability, enhanced oral bioavailability, and improved blood-brain barrier penetration.
Molecular Glue-Antibody Conjugates (MACs)
As research into molecular glue degraders advances, scientists are exploring strategies to enhance their selectivity and reduce systemic toxicity. Drawing inspiration from ADCs and degrader-antibody conjugates (DACs), researchers have developed a new class of therapeutics—Molecular Glue-Antibody Conjugates (MACs)—that combine the precise targeting capability of antibodies with the catalytic protein degradation mechanism of molecular glues.

Figure 1. MAC advantages[1]
By integrating the complementary advantages of ADCs and molecular glue degraders, MACs successfully overcome the inherent limitations of both approaches. Their innovation can be summarized in several key aspects:
- ● Antibody-mediated targeted delivery: Conjugation of molecular glues to antibodies enables precise delivery to cancer cells, significantly enhancing target specificity and reducing off-target effects.
- ● Optimized pharmacokinetics: The antibody component facilitates cellular uptake of the molecular glue and prolongs its half-life, effectively improving the bioavailability that free degraders often lack.
- ● Improved therapeutic index: Compared with free molecular glues, MACs achieve comparable degradation activity at much lower systemic concentrations. This widens the therapeutic window, enhances tolerability, and minimizes adverse effects.
- ●Dual-precision targeting: By combining the substrate selectivity of molecular glues with the antigen specificity of antibodies, MACs achieve dual precision—selectively recognizing tumor cells while degrading disease-causing proteins—thus further reducing off-target toxicity.
Global MAC Pipelines Overview
Although there are currently only around ten MAC candidates in development worldwide, leading biotech companies have already demonstrated clear directions for technological breakthroughs. Several of these programs have even advanced into clinical validation, marking the beginning of a new phase for this emerging modality.
Orum Therapeutics
As one of the pioneers in the MAC field, Orum Therapeutics initially developed ORM-5029, its first clinical-stage MAC, but later discontinued that program. The company continues to advance three core pipelines — ORM-6151 (BMS-986497), ORM-1023, and ORM-1153.
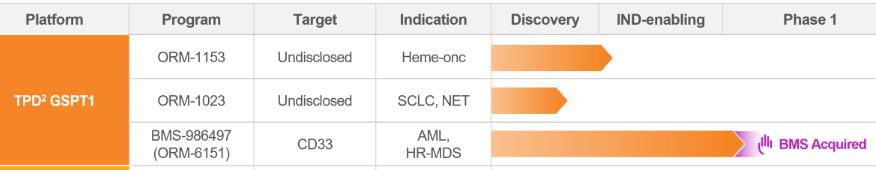
Figure 2. MAC Pipelines of Orum Therapeutics, source: https://www.orumrx.com/pipeline-page
ORM-6151 is a first-in-class anti-CD33 antibody-enabled GSPT1 degrader that has received FDA clearance for a Phase I trial in patients with acute myeloid leukemia (AML) or high-risk myelodysplastic syndromes (MDS).
Medilink / Kintor
Medilink / Suzhou Kintor Therapeutics has disclosed the development of a series of molecular glue-antibody conjugates (MACs)—MAC-001, MAC-002, and MAC-003—designed to degrade the c-Myc protein using CRBN-based molecular glues (D-1, D-2, and D-10) as payloads.
The study employed mesylpyrimidine linkers to construct high drug-to-antibody ratio (DAR = 8) MACs using either VKG or VC-PAB as the conjugation linker. Among these candidates, MAC-002 demonstrated the most potent activity, achieving a 72% tumor growth inhibition rate in NOD/SCID mouse efficacy models.
Legochem Biosciences
Legochem Biosciences has developed a β-glucuronide linker-based, two-step conjugation strategy that allows site-specific coupling of molecular glues to engineered antibodies, resulting in MAC molecules with a DAR of 2.
This precise conjugation method significantly enhances homogeneity and stability, providing a new approach for optimizing pharmacokinetic properties. Preclinical studies have shown that Legochem’s MAC candidates exhibit strong tumor accumulation and promising efficacy in solid tumor models.
Summary and Outlook
MACs are not just an incremental advancement—they mark a paradigm shift in therapeutic design. By combining the targeting capabilities of ADCs with the catalytic degradation power of molecular glues, MACs go beyond traditional payload-based cytotoxicity. This fusion enables the degradation of previously “undruggable” intracellular targets, expanding the therapeutic potential far beyond surface antigens.
References:
[1] Tao Y, Lu Y, Yu B, Wang Y. Molecular glue meets antibody: next-generation antibody-drug conjugates. Trends Pharmacol Sci. 2025 Jun;46(6):520-534. doi: 10.1016/j.tips.2025.04.002. Epub 2025 May 8. PMID: 40345868.
[2] Poudel YB, Thakore RR, Chekler EP. The New Frontier: Merging Molecular Glue Degrader and Antibody-Drug Conjugate Modalities To Overcome Strategic Challenges. J Med Chem. 2024 Sep 26;67(18):15996-16001. doi: 10.1021/acs.jmedchem.4c01289. Epub 2024 Sep 4. Erratum in: J Med Chem. 2024 Nov 14;67(21):19925. doi: 10.1021/acs.jmedchem.4c02447. PMID: 39231796.
[3] Guo, Y., Song, Y., Wang, H., Lu, Y., Zhang, J., Shen, Z., Kan, W., Wang, Y., Duan, H., Geng, S., Wang, B., Li, S., Li, B., Chen, X., Pei, S., Fang, L., Li, J., Zhou, Y., Che, J., . . . Dong, X. (2025). Rational engineering of degradation tail-driven CELMoD-antibody conjugates for precision malignancy therapy. Acta Pharmaceutica Sinica B. https://doi.org/10.1016/j.apsb.2025.09.009

