Recently, tumor immunotherapy has developed rapidly, especially the combined protocol of PD-1/PD-L1 inhibitors and chemotherapy, which has become a new standard of treatment for many cancers. However, the systemic toxicity caused by chemotherapy are still a pressing clinical challenge.
Antibody Drug Conjugates (ADCs) can effectively improve the therapeutic window of chemotherapeutic drugs. Therefore, the design of PD-L1-directed ADC drugs is also a highly promising direction for research and development.
ADC & Immune Checkpoint Inhibitors (ICIs)
ADC drugs are novel tumor-targeted therapeutics consisting of antibody, linker and payload, which integrate the chemotherapeutic drug toxicity of payload and antibody-targeting effects. In recent years, with the breakthrough of coupling technology and the introduction of novel linkers and payloads, ADC drug development has gradually entered a fast track. Currently, 15 ADCs have been approved and more than 100 products are in the clinical stage.

Figure 1. FDA Approved ADCs, source: reference [2]
In recent years, immune checkpoint inhibitors (ICIs) represented by PD-1 and PD-L1 antibodies have promoted the rapid development of tumor immunotherapy and become another effective tumor treatment after surgery, radiotherapy, chemotherapy and targeted therapy. Currently, several PD-L1 monoclonal antibodies have been approved for marketing worldwide, including durvalumab (Imfinzi) from AstraZeneca, atezolizumab (Tecentriq) from Roche, and avelumab (Bavencio) from Merck/Pfizer.
However, the heterogeneity of tumors and the complexity of the regulatory mechanism of PD-L1 expression, as well as the poor penetration of large-molecule anti-PD-L1 monoclonal antibodies (mAbs) into dense tumors, have led to a great limitation of the clinical application of anti-PD-L1 m, with the overall objective response rate of patients at around 15%-30%.
To improve response rates and treatment outcomes in oncology patients, anti-PD-1 mAbs have been explored in combination with chemotherapy, targeted therapies and radiotherapy, especially chemotherapy. Since chemotherapy can lead to the death of immunogenic cells, thus allowing more tumor antigens to be released and effectively promoting the activation of immune response. In addition, it can reduce the number and activity of immunosuppressive cells. Therefore, chemotherapy is the main option for immune combination. However, the systemic side effects caused by chemotherapy remain a thorny issue.

Figure 2. ADC Proposed Mechanism of Action, source: https://abbviescience.com/
Given that PD-L1 is expressed in multiple tumor types and has limited expression in normal tissues, combining ADC drugs with anti-PD-L1 mAbs to generate bifunctional PD-L1-directed ADCs would be a very promising development direction.
Research of PDL1 ADC
Currently, there are several PD-L1-targeted ADC drugs in preclinical and Phase I stages. Among them, Seagen's SGN-PDL1V has made the fastest progress.
| Drug | Target | Type | Company | Indication | Phase |
| SGN-PDL1V | PDL1 | ADC | Seagen | Solid tumors | Phase I |
| 18F-BMS-986229 | PDL1 | PDC | BMS | Adenocarcinoma of esophagogastric junction | Unknown |
| AB003 | PDL1,PDL2 | ADC | AKSO | Solid tumors | Preclinical |
| Anti PD-L1 ISAC | PDL1 | ISAC | Bolt Biopharmaceutical Inc | Tumors | Preclinical |
| Anti PD-L1 Immunoconjugate | PDL1 | ADC | IGM Biosciences | Hemooncology, Solid tumors | Preclinical |
| Atezolizumab ADC | PDL1 | ADC | Beijing Institute of Pharmacology and Toxicology | Renal cell carcinoma | Preclinical |
| Boltbody ISAC PD-L1 | PDL1, TLR8, TLR7 | ADC | Bolt Biopharmaceutical Inc | Solid tumors | Preclinical |
| BDB101 | PDL1 | ADC | Seven and Eight Biopharmaceuticals | Solid tumors | Preclinical |
| HE-S2 | PDL1 | ADC | Tsinghua University | Tumors | Preclinical |
| IMGS-501 | STING, PDL1, PDL2 | ADC | ImmunoGenesis | Tumors | Preclinical |
| YB1-ADC-PDL1 | PDL1 | ADC | HKND YB1 PHARMACEUTUCAL | Melanoma | Preclinical |
Table: PDL1-ADC pipeline
SGN-PDL1V is a PD-L1-directed ADC currently under preclinical investigation that consists of an anti-PD-L1 antibody conjugated to a vedotin drug ligand. The vedotin drug linker consists of a microtubule disruptor, monomethyl auristatin E (MMAE), and a protease-cleavable peptide linker. The main mechanism of action of the proposed SGN-PDL1V is direct cytotoxicity against PD-L1-expressing malignant cells by delivering the MMAE payload. In addition, MMAE induces immunogenic cell death leading to subsequent immune activation of the tumor microenvironment.

Figure 3. SGN-PDL1V mechanism of action, source: 2021SITC
Data from preclinical investigation of SGN-PDL1V were presented during the Society for Immunotherapy of Cancer (SITC) annual meeting in 2021.
1) SGN-PDL1V exhibits high endocytosis rate and strong cytotoxic killing activity
- ▶ Fast endocytosis into cells: Seagen PD-L1 mAb enables faster cellular internalization and protein hydrolysis than other anti-PD-L1 mAbs that have been approved for marketing.
- ▶ In vitro cytotoxic activity against cancer cells: consistent with internalization data, SGN-PDL1V was effective in drug delivery and induced cytotoxicity in PD-L1-positive cancer cells, while no relevant activity was produced in PD-L1-negative cells.
2) SGN-PDL1V has PD-1/PD-L1 immune checkpoint blocking potential
- ▶ SGN-PDL1V blocked PD-1/PD-L1 checkpoint pathway in vitro: SGN-PDL1V and SeagenPD-L1 mAb without payload had comparable blocking efficiency for PD-1/PD-L1 checkpoint pathway, indicating that the blocking activity of PD-1/PD-L1 checkpoint pathway was not affected after design as ADC.
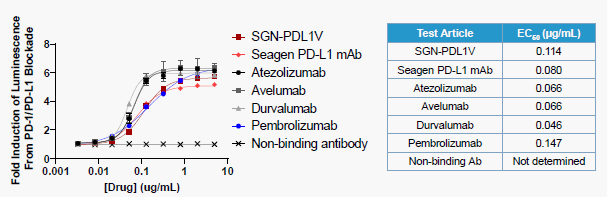
Figure 4. The detection of PD-1/PD-L1 checkpoint pathway blocking activity
- ▶ In vivo antitumor activity of Seagen PD-L1 mAb suggests PD-1/PD-L1 checkpoint blocking activity: The immunoreactive MC38 model expressing humanized PD-L1 enables the evaluation of PD-1/PD-L1 checkpoint blocking activity. (Procedure: MC38 cells expressing humanized PD-L1 were subcutaneously implanted into female C57BL/6 mice expressing human extracellular structural domain chimeric PD-1 and PD-L1. When the tumor volume reached 100-150 mm3, the donor was injected intravenously every 3 days for a total of 3 times. (Each treatment group included 10 mice.)
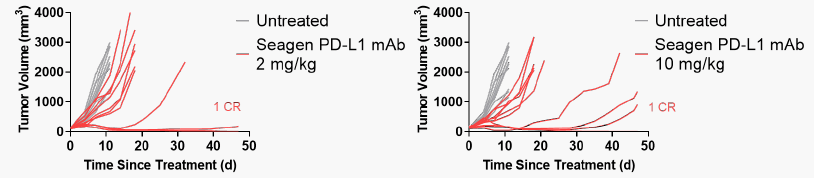
Figure 5. Seagen PD-L1 mAb anti-tumor activity trial
Seagen is also currently developing models capable of simultaneously assessing the cytotoxicity of SGN-PDL1V and validating the mechanism of action of checkpoint blockade.
Based on data from preclinical investigations, Seagen has initiated a Phase I clinical trial (NCT 05208762) in 2022 to evaluate the safety of SGN-PDL1V in patients with solid tumors (NSCLC, head and neck squamous cell carcinoma, esophageal cancer, ovarian cancer, melanoma). The study is divided into 3 parts: Parts A and B will explore the dosing of SGN-PDL1V; Part C will evaluate the safety of SGN-PDL1V using the dosing determined in Parts A and B. It is planned to enroll 305 patients. This shows the great expectations of Seagen for SGN-PDL1V.

Figure 6. PHASE 1 STUDY OF SGN-PDL1V, source: https://seagenmedicalaffairs.com/document/pdl1v-solid-tumors-tps3154
In addition to SGN-PDL1V, which has entered the phase I clinical stage, there are several PDL1-ADC products in the preclinical stage, with the main differences being the choice of linker, payload, and antibody.
Selection of Linker
Some research teams have started to try to make a breakthrough in linker selection. Since the tumor extracellular environment is inherently acidic, acid pH sensitive linkers are also often used in ADC design so that payload (e.g. Dox, epirubicin) can be selectively released in the tumor environment.

Figure 7. Mechanism of PDL1-Dox ADC, source: reference [5]
The team introduced the PDL1-Dox ADC containing a hydrazone linker that will selectively cleave in the tumor cell environment. In addition, the team used PEG spacers to enhance the water solubility of the antibody and maintain the circulation of PDL1-Dox.
Selection of Antibody
In terms of antibody selection, some research teams have also chosen Atezolizumab, an anti-PD-L1 mAb that has been approved for marketing. The design idea is that Atezolizumab generates bifunctional PD-L1-ADC by binding to MMAE through a classical dipeptide (valine - alanine) linker.

Figure 8. Mechanism of Bifunctional PD-L1-targeted ADC, source: reference [3]
Atezolizumab was found to have higher endocytosis efficiency compared to avelumab through pre-basic studies, making it an ideal antibody choice for PD-L1-ADC products.
Conclusion
With the continuous advancement of tumor therapies, the concept of multi-target and multi-mechanism synergistic anti-tumor has gradually become a direction of anti-tumor drug development.
In view of the breakthroughs achieved by ADCs and ICIs in tumor therapy in recent years, a number of companies have already started to explore the combination of ADCs and PD-1/L1 inhibitors and have achieved encouraging efficacy data.
Biopharma PEG, as a professional PEG supplier, offers a variety of PEG linkers to facilitate your drug development, such as antibody-drug conjugate (ADC), peptide drug conjugates (PDC), etc. All PEG linkers are of >95% purity and they are the basic building blocks for a successful ADC.
References:
[1] Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther. 2022;7(1):93. Published 2022 Mar 22. doi:10.1038/s41392-022-00947-7
[2] Tarantino P, Carmagnani Pestana R, Corti C, et al. Antibody-drug conjugates: Smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 2022;72(2):165-182. doi:10.3322/caac.21705
[3] Xiao D, Luo L, Li J, et al. Development of bifunctional anti-PD-L1 antibody MMAE conjugate with cytotoxicity and immunostimulation. Bioorg Chem. 2021;116:105366. doi:10.1016/j.bioorg.2021.105366
[4] Nagaya T, Nakamura Y, Sato K, Harada T, Choyke PL, Hodge JW, Schlom J, Kobayashi H. Near infrared photoimmunotherapy with avelumab, an anti-programmed death-ligand 1 (PD-L1) antibody. Oncotarget. 2017 Jan 31;8(5):8807-8817. doi: 10.18632/oncotarget.12410. PMID: 27716622; PMCID: PMC5341755.
[5] Sau S, Petrovici A, Alsaab HO, Bhise K, Iyer AK. PDL-1 Antibody Drug Conjugate for Selective Chemo-Guided Immune Modulation of Cancer. Cancers (Basel). 2019;11(2):232. Published 2019 Feb 16. doi:10.3390/cancers11020232
Related Articles:
Bispecific Antibody Drug Conjugates (ADCs): Emerging Trends
Molecular Glues: A New Dawn After PROTAC
Overview of PEG Linkers & Their Applications

