Nucleoside-modified messenger RNA (mRNA), delivered through lipid nanoparticles (LNPs), laid the foundation for the first COVID-19 vaccines authorized for emergency use. Since then, mRNA has quickly moved beyond vaccines and is now viewed as a versatile tool for therapy, prevention, and diagnostics. One of its strongest advantages is the ability to precisely regulate immune cells such as T lymphocytes, creating new possibilities for treating cancer, infectious diseases, and immune-related conditions.
Even with its potential, delivery remains the biggest obstacle. After systemic administration, most mRNA-LNPs accumulate in the liver. This limits their effectiveness against diseases in other tissues, including tumors, the lungs, the heart, and the central nervous system. A common workaround is increasing the dosage, but higher exposure can trigger immune toxicity and reduce tolerability. Without better targeting, many promising mRNA applications remain out of reach.
To overcome these barriers, researchers are developing antibody-targeted lipid nanoparticles (Ab-LNPs). By attaching specific antibodies to the nanoparticle surface, Ab-LNPs are designed to navigate beyond the liver and home in on desired cell types or tissues. This strategy uses the natural targeting ability of antibodies to guide nanoparticles through complex biological environments.
The concept behind antibody-targeted lipid nanoparticles (Ab-LNPs) is similar to that of antibody-drug conjugates (ADCs). In this approach, an antibody or antibody-like molecule serves as the targeting component. By binding to specific receptors on the surface of target cells, the antibody directs RNA-loaded LNPs to the intended site of action. The targeting unit can take many forms, including monoclonal antibodies, bispecific antibodies, single-domain antibodies, peptides, or apolipoproteins. This flexibility allows Ab-LNPs to be tailored for different disease settings and delivery goals.
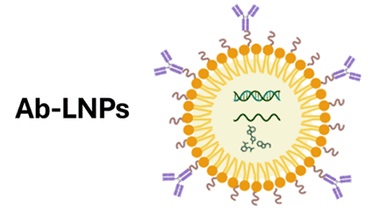
Figure 1. AB-LNP [1]
Key Advantages Over Conventional mRNA-LNPs
Compared with standard RNA-LNP formulations, Ab-LNPs offer three main advantages:
1. Higher therapeutic effectiveness
Because the antibody binds selectively to target cells, the LNPs are delivered where they are needed instead of dispersing into non-target tissues. This improves cellular uptake and enhances treatment outcomes.
2. Reduced toxicity
Targeted delivery helps limit accumulation in organs such as the liver. By lowering off-target exposure, Ab-LNPs may reduce risks such as acute liver toxicity and systemic side effects.
3. Broad applicability
By switching the antibody or binding domain, Ab-LNPs can be optimized for different receptors and cell types. This makes them suitable for areas such as lymphoma immunotherapy, vaccine development, and other RNA-based interventions.
Besides, the exterior of the LNP can be modified to improve performance. For example, incorporating polyethylene glycol (PEG) can extend circulation time and enhance particle stability. Additional functional molecules may also be added to further improve targeting precision and therapeutic activity.
Clinical Progress of Ab-LNPs
Ab-LNPs are rapidly moving from concept to clinical reality, especially in the field of in vivo CAR-T therapy. Their ability to deliver mRNA directly to immune cells makes them one of the most promising delivery platforms under development.
A major milestone came on June 30, 2025, when AbbVie acquired Capstan Therapeutics for $2.1 billion. Capstan is considered a rising player in in vivo CAR-T technology, and its lead candidate, CPTX2309, a potential first-in-class in vivo tLNP anti-CD19 CAR-T therapy candidate. The LNP was modified with maleimide functioning groups (DSPE-PEG-mal). It is currently in Phase I clinical development for B cell–mediated autoimmune disorders. [2]
CPTX2309 is built on Capstan’s proprietary platform, which is designed to avoid unwanted accumulation in the liver. The therapy delivers mRNA that encodes an anti-CD19 CAR directly to CD8-expressing cytotoxic T cells. Unlike traditional CAR-T approaches, this process happens entirely in vivo. It does not require lymphodepletion or the complex ex vivo cell manufacturing used in conventional treatments.
Once inside the body, the modified CD8+ T cells temporarily express the CD19 CAR and target B cells in both tissues and circulation. By clearing autoreactive, antibody-producing memory B cells and allowing naïve B cells to repopulate, the therapy aims to “reset” the immune system. This strategy has the potential to stop disease progression and support long-term remission.
Breakthroughs from the Drew Weissman Lab in Ab-LNPs
In the field of Ab-LNP delivery, few teams have made contributions as impactful as Dr. Drew Weissman’s. Weissman, who received the 2023 Nobel Prize in Physiology or Medicine and now leads the Penn RNA Innovation Institute, is best known for developing the nucleoside-modified mRNA platform. This breakthrough helped prevent unwanted immune reactions and paved the way for the first COVID-19 mRNA vaccines. It also created the technical foundation for today’s Ab-LNP innovations.
Weissman’s group has pushed this platform in three key targeting directions, with each breakthrough published in leading journals.
1. Targeting the Lungs: Anti-PECAM-1/mRNA-LNP
A major limitation of conventional mRNA delivery is that it defaults to the liver and rarely reaches other organs effectively. By conjugating antibodies to the LNP surface, delivery can be redirected with far greater precision.
Weissman’s team demonstrated that decorating LNPs with an anti-PECAM-1 antibody dramatically changed biodistribution in mice after IV injection. Compared to untargeted LNPs, Ab-LNP mRNAs resulted in profound inhibition of hepatic uptake concomitantly with ~200-fold and 25-fold elevation of mRNA delivery and protein expression in the lungs. Notably, this process did not rely on apolipoprotein E (APOE), which is essential for liver-targeted delivery in traditional LNPs. This approach opens new opportunities for treating cardiovascular, neurological, and pulmonary diseases using mRNA. [3]
2. Precision Delivery to CD4+ T Cells: Anti-CD4/mRNA-LNP
Most cellular immunotherapies today depend on complex protein drugs or ex vivo engineering steps, as seen in conventional CAR-T treatments. These approaches are time-consuming, costly, and carry risks such as insertional mutagenesis.
Weissman’s lab designed anti-CD4 antibody–modified mRNA-LNPs that can efficiently and selectively deliver mRNA into cultured CD4+ T cells. Compared with non-targeted LNPs, reporter gene expression in these cells jumped roughly 30-fold. When loaded with Cre recombinase mRNA, the system enabled dose-dependent loxP-mediated gene recombination. Delivery was consistent across naïve, central memory, and effector memory CD4+ T-cell subtypes. This work lays a strong foundation for mRNA-based T-cell immunotherapies in cancer, HIV, and other diseases. [4]
3. Targeting Bone Marrow and HSCs: Anti-CD117/LNP-mRNA
Hematopoietic stem cell (HSC) transplantation remains a cornerstone for treating many blood disorders, but current conditioning regimens rely on radiation or chemotherapy, which introduce serious long-term toxicity.
To address this, the team developed anti-CD117 antibody–modified LNPs that selectively target bone marrow cells and HSCs. Two major applications emerged:
l Gene correction: When loaded with base-editing mRNA, the system was able to nearly fully correct the sickle cell disease mutation (E6V) in vitro.
l Selective conditioning: When loaded with mRNA encoding PUMA (a pro-apoptotic protein regulated by p53), the particles eliminated diseased HSCs in vivo. This offers a conditioning strategy free from genotoxic damage, potentially transforming transplant safety.
These results not only extend Ab-LNP use into hematologic diseases but also point toward in vivo genome editing as a treatment path for inherited conditions.
Biopharma PEG is a leading worldwide PEG supplier that dedicated to manufacturing and supplying kilogram scale manufacture of PEG derivatives in both GMP and non-GMP grades, including monodispersed PEGs, polydispersed PEGs and multi-arm PEGs, etc.
References:
[1] Unleashing the Potential: Designing Antibody-Targeted Lipid Nanoparticles for Industrial Applications with CMC Considerations and Clinical Outlook, Sheryl Wang, Hong Wang, Andrew Drabek, Wenwen Sha Smith, Feng Liang, and Zhaohua Richard Huang, Molecular Pharmaceutics 2024 21 (1), 4-17, DOI: 10.1021/acs.molpharmaceut.3c00735
[2]
https://news.abbvie.com/2025-06-30-AbbVie-to-Acquire-Capstan-Therapeutics,-Further-Strengthening-Commitment-to-Transforming-Patient-Care-in-Immunology
[3] Parhiz H, Shuvaev VV, Pardi N, Khoshnejad M, Kiseleva RY, Brenner JS, Uhler T, Tuyishime S, Mui BL, Tam YK, Madden TD, Hope MJ, Weissman D, Muzykantov VR. PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake. J Control Release. 2018 Dec 10;291:106-115. doi: 10.1016/j.jconrel.2018.10.015. Epub 2018 Oct 15. PMID: 30336167; PMCID: PMC6477695.
[4] Tombácz I, Laczkó D, Shahnawaz H, Muramatsu H, Natesan A, Yadegari A, Papp TE, Alameh MG, Shuvaev V, Mui BL, Tam YK, Muzykantov V, Pardi N, Weissman D, Parhiz H. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol Ther. 2021 Nov 3;29(11):3293-3304. doi: 10.1016/j.ymthe.2021.06.004. Epub 2021 Jun 4. PMID: 34091054; PMCID: PMC8571164.
[5] Breda L, Papp TE, Triebwasser MP, Yadegari A, Fedorky MT, Tanaka N, Abdulmalik O, Pavani G, Wang Y, Grupp SA, Chou ST, Ni H, Mui BL, Tam YK, Weissman D, Rivella S, Parhiz H. In vivo hematopoietic stem cell modification by mRNA delivery. Science. 2023 Jul 28;381(6656):436-443. doi: 10.1126/science.ade6967. Epub 2023 Jul 27. Erratum in: Science. 2025 Jun 5;388(6751):eadz0744. doi: 10.1126/science.adz0744. PMID: 37499029; PMCID: PMC10567133.
Related Articles:
In Vivo CAR-T Delivery: mRNA-LNP System
Application of mRNA-Lipid Nanoparticles (LNPs) In Cancer
Nucleic Acid Therapeutics: Approvals and Potential Blockbusters
The Applications and Challenges of Lipid Nanoparticles
The Role of Four Lipid Components Of LNPs

