Multiple myeloma (MM) is a malignant proliferation of plasma cells (PCs) that produces excess monoclonal immunoglobulins, resulting in a series of clinical manifestations including extensive bone destruction, recurrent infections, anemia, hypercalcemia, hyperviscosity syndrome and renal insufficiency.
Although multiple myeloma remains an incurable disease, the 5-year relative survival rate has nearly doubled in the last 20 years, from 32.1% in 1996 to 54.9% in 2016, according to the National Cancer Institute. Over the past 5-10 years, numerous treatment options have entered the myeloma treatment arena, greatly extending progression-free survival (PFS) and overall survival (OS). The focus of research has shifted from systemic therapies such as chemotherapy to more specific targeted therapies such as immunotherapy. A variety of innovative targeted therapies that are effective against multiple myeloma, such as monoclonal antibodies (mAbs), CAR-T cell therapy, antibody-drug conjugates(ADCs), and bispecific antibodies, have made rapid progress.
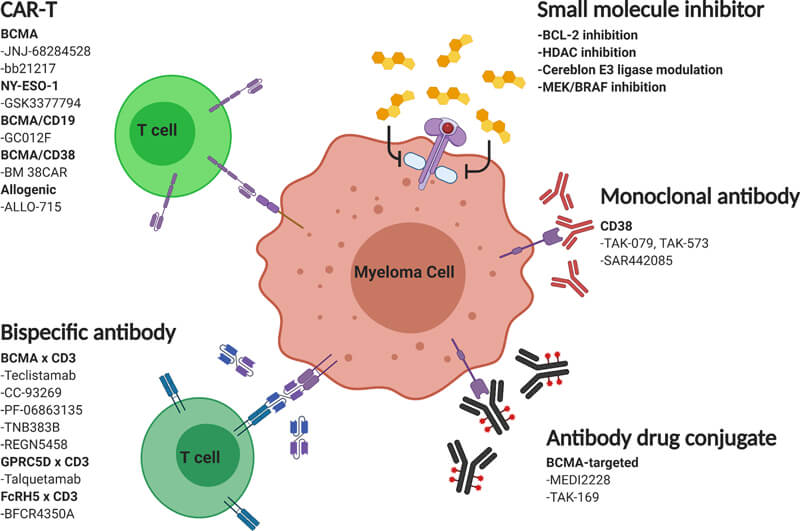
Figure 1. Mechanisms of action of emerging multiple myeloma therapies, Source: reference 1
Monoclonal Antibodies (mAbs)
The first immunotherapies approved for use in patients with multiple myeloma are the monoclonal antibodies Daratumumab (Darzalex) and Elotuzumab (Empliciti). The third monoclonal antibody, Isatuximab (Sarclisa), was approved for relapsed refractory multiple myeloma in March 2020.
| Drug | Target | Company | Approval Date |
| Daratumumab | CD38 | Johnson & Johnson/Genmab | Nov 2015(FDA) |
| Elotuzumab | SLAMF7 | BMS/AbbVie | Nov 2015(FDA) |
| Isatuximab | CD38 | Sanofi | Mar 2020 (FDA) |
Daratumumab
Daratumumab, a humanized, anti-CD38 IgG1 monoclonal antibody developed by Johnson & Johnson in collaboration with Genmab, binds to CD38 expressed by tumor cells and induces apoptosis through various immune-related mechanisms, including complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), as well as Fcγ receptors.
Unlike traditional targeted drugs, Daratumumab is the first monoclonal antibody drug that directly targets the CD38 protein on multiple myeloma cells. In November 2015, Daratumumab was approved for marketing by the U.S. Food and Drug Administration (FDA) for the treatment of multiple myeloma in people who had received at least three prior therapies.
Elotuzumab
Elotuzumab is a monoclonal antibody developed by Bristol-Myers Squibb (BMS) and AbbVie that targets the cell surface protein SLAMF7, which is found in both multiple myeloma cells and natural killer (NK) cells. It provides a dual attack on cancer by directly targeting multiple myeloma cells and enhancing the ability of NK cells to kill them. The drug did not have significant activity alone in the treatment of relapsed/refractory multiple myeloma, but when combined with other antitumor agents, such as bortezomib or lenalidomide, the antimyeloma activity was significant, improving patient response rates and clinical prognosis. Elotuzumab was approved for marketing by the FDA on November 30, 2015, and was used in combination with lenalidomide and dexamethasone in combination with lenalidomide and dexamethasone for the treatment of multiple myeloma with one to three prior treatments.
Isatuximab
Isatuximab, an IgG1 chimeric monoclonal antibody developed by Sanofi, targets a specific epitope of the plasma cell CD38 receptor and is able to trigger a variety of unique mechanisms of action, including promotion of programmed tumor cell death (apoptosis) and immunomodulatory activity. CD38 is expressed at high levels on multiple myeloma cells and is a cell surface receptor target for antibody therapy in multiple myeloma and other malignancies.
Isatuximab has been granted orphan drug status for the treatment of relapsed/refractory multiple myeloma in both the United States and the European Union. Currently, related studies are also evaluating the potential of Isatuximab in other hematologic malignancies and solid tumors. On March 2, 2020, the U.S. FDA approved Isatuximab in combination with carfilzomib and dexamethasone, for the treatment of adult patients with relapsed or refractory multiple myeloma who have received one to three prior lines of therapy.
CAR T-cell Therapies
Another immunotherapy targeting multiple myeloma is against another target on the surface of myeloma cells, the B-cell maturation antigen (BCMA), which is not found in healthy cells. The most promising of the BCMA-targeted therapies is CAR-T cell therapy.

Figure 2. The two CAR T-cell therapies approved by FDA for treating multiple myeloma bind to the BCMA protein (blue) on the surface of myeloma cells. Credit: Adapted from Frontiers in Immunology. August 2018. https://doi.org/10.3389/fimmu.2018.01821. CC BY 4.0.
CAR-T therapy targeting myeloma-associated antigens offers new hope for a cure for MM, and two BCMA CAR-T cell therapies have been approved by the FDA for patients with relapsed/refractory multiple myeloma who have progressed after 4 or more lines of therapy, including Idecabtagene Vicleucel (ide-cel) and cidagiorenza (cilta-cel). The ORR of ide-cel treatment in the previous median 6 lines was 73%, the MRD negative rate was 26%, and the median OS was 24.8 months; the ORR of cilta-cel treatment in the previous median 5 lines was 97%, the MRD negative rate was 93%, and the 12-month OS rate was 89%. From these data, it is easy to see that CAR-T therapy has a good clinical effect in the treatment of multiple myeloma.
Cytokine release syndrome (CRS) and neurotoxicity are common adverse effects of CAR-T cell therapy, independent of the target or tumor type. In clinical studies, the majority of CRS events were grade 1 or 2, with only a very small number of patients being grade ≥3. Most CRS can be treated with tocilizumab and other standard approaches. Most neurotoxic events were manageable and manageable.
CAR-T is a promising new approach for the treatment of MM with the ability to kill MM cells that are resistant to standard therapies. CAR-T for MM is currently in the improvement and enhancement phase, and the main goals for the future are to improve CAR-T structure, increase CAR-T safety timeliness, and manage long-term complications of CAR-T cell therapy.
Antibody Drug Conjugates (ADCs)
Antibody–drug conjugates consist of recombinant monoclonal antibodies covalently bound to cytotoxic chemicals (known as warheads or payload) via synthetic linkers.
In August 2020, the U.S. FDA granted accelerated approval to Blenrep (belantamab mafodotin) from GlaxoSmithKline (GSK) as monotherapy for adult patients with relapsed or refractory multiple myeloma who have received at least four prior therapies, including anti-CD38 monoclonal antibodies, proteasome inhibitors, and immunomodulators. Notably, Blenrep is the world's first approved BCMA-directed antibody-drug conjugate.
The approval of Blenrep is based on the results of the DREA multiple myeloma clinical trial program, which enrolled patients with R/R multiple myeloma whose disease was still progressing after current standard therapy. In the study, patients received single-agent Blenrep 2.5 mg/kg administered once every 3 weeks. The results of the study showed that patients (n=97) treated with Blenrep who received both median 7 lines of therapy achieved an overall response rate (ORR) of 31% (97.5% CI; 21-43); median duration of response (DoR) had not been reached at the 6-month analysis, however, 73% of these patients in remission had a DoR equal to or greater than 6 months. The most common adverse events were keratoconus (20%), vision loss, nausea, blurred vision, fever, infusion-related reactions, and fatigue. Compared to CAR-T therapies, ADCs are inexpensive and easy to manufacture and administer. They also do not cause cytokine release syndrome.
There are many ADCs under investigation for MM, including Lorvotuzumab Mertansine, HDP-101, Anti-ICAM1, AMG 224, ABBV-838, Anti-FcRH5, etc. (Table 1)

Table 2. ADCs ongoing trails in MM, source: reference 2
Bispecific Antibodies (BiAbs)
Bispecific Antibodies represent another approach to treating MM, utilizing the high cytolytic activity of T cells. While one arm binds to a plasma cell or B-cell lineage-associated antigen, a second arm recruits T cells via the CD3 domain, thereby bringing T cells in close proximity to MM cells, ultimately leading to granzyme and perforin exocytosis and apoptosis of the target cell. Now, there are no bispecific antibodies approved for MM, but many drugs are under investigation, such as Teclistamab, Pavurutamab (AMG 701), CC-93269, AMG 420, PF-3135, REGN5458, Talquetamab, etc.

Table 2. Bispecific antibodies ongoing trails in MM, source: reference 2
A key benefit of bispecific antibodies is that they do not require conditional chemotherapy prior to treatment. In contrast, a person receiving CAR-T cells must first receive lymphocyte-reducing chemotherapy to give the CAR-T cells the best chance of fighting cancer. Unlike CAR-T cells, bispecific antibodies do not need to be made specifically for each individual; they are off-the-shelf preparations that can be used immediately. However, bispecific antibodies do have some of the same side effects as CAR-T cell therapy, including cytokine release syndrome and neurotoxicity.
Conclusion
According to Datamonitor Healthcare forecasts, the combined size of single-disease mainstream drugs for multiple myeloma in major countries worldwide (seven countries, including the United States, Japan, France, Germany, Italy, Spain, and the United Kingdom) is approximately $12 billion, with a steady growth rate of 5-8% over the next 3-5 years. The current practice for MM treatment involves immune therapy as a standard approach for RRMM, and it is increasingly being used for NDMM and studies have shown promising results. We are looking forward to more innovative drugs approved for MM.
Biopharma PEG provides GMP standard PEG derivatives and bulk orders via custom synthesis, offering the opportunity to match customers' special quality requirements. ADC linkers with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis.
References:
1. Su, C.T., Ye, J.C. Emerging therapies for relapsed/refractory multiple myeloma: CAR-T and beyond. J Hematol Oncol 14, 115 (2021). https://doi.org/10.1186/s13045-021-01109-y
2. Tai W, Wahab A, Franco D, et al. Emerging Role of Antibody-Drug Conjugates and Bispecific Antibodies for the Treatment of Multiple Myeloma. Antibodies (Basel). 2022;11(2):22. Published 2022 Mar 24. doi:10.3390/antib11020022
3. B cell maturationantigen (BCMA)-based immunotherapy for multiple myeloma. DOI: 10.1080/14712598.2019.1641196
Related Articles:
Global Antibody-drug Conjugates (ADCs): Approvals & Clinical Trails Review
Recent Advances In Hematology 2022: ADCs, Bispecific Antibodies, CAR-T Cells…
Bispecific Antibodies - Current Status and Prospects

